Pseudomonas evades immune recognition of flagellin in both mammals and plants
- PMID: 21901099
- PMCID: PMC3161968
- DOI: 10.1371/journal.ppat.1002206
Pseudomonas evades immune recognition of flagellin in both mammals and plants
Abstract
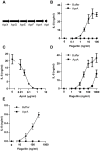
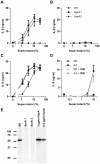
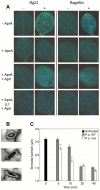
The building blocks of bacterial flagella, flagellin monomers, are potent stimulators of host innate immune systems. Recognition of flagellin monomers occurs by flagellin-specific pattern-recognition receptors, such as Toll-like receptor 5 (TLR5) in mammals and flagellin-sensitive 2 (FLS2) in plants. Activation of these immune systems via flagellin leads eventually to elimination of the bacterium from the host. In order to prevent immune activation and thus favor survival in the host, bacteria secrete many proteins that hamper such recognition. In our search for Toll like receptor (TLR) antagonists, we screened bacterial supernatants and identified alkaline protease (AprA) of Pseudomonas aeruginosa as a TLR5 signaling inhibitor as evidenced by a marked reduction in IL-8 production and NF-κB activation. AprA effectively degrades the TLR5 ligand monomeric flagellin, while polymeric flagellin (involved in bacterial motility) and TLR5 itself resist degradation. The natural occurring alkaline protease inhibitor AprI of P. aeruginosa blocked flagellin degradation by AprA. P. aeruginosa aprA mutants induced an over 100-fold enhanced activation of TLR5 signaling, because they fail to degrade excess monomeric flagellin in their environment. Interestingly, AprA also prevents flagellin-mediated immune responses (such as growth inhibition and callose deposition) in Arabidopsis thaliana plants. This was due to decreased activation of the receptor FLS2 and clearly demonstrated by delayed stomatal closure with live bacteria in plants. Thus, by degrading the ligand for TLR5 and FLS2, P. aeruginosa escapes recognition by the innate immune systems of both mammals and plants.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
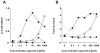



References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. - PubMed
-
- Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–1253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

