Targeted disruption of TgPhIL1 in Toxoplasma gondii results in altered parasite morphology and fitness
- PMID: 21901148
- PMCID: PMC3162014
- DOI: 10.1371/journal.pone.0023977
Targeted disruption of TgPhIL1 in Toxoplasma gondii results in altered parasite morphology and fitness
Abstract
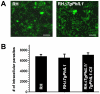
The inner membrane complex (IMC), a series of flattened vesicles at the periphery of apicomplexan parasites, is thought to be important for parasite shape, motility and replication, but few of the IMC proteins that function in these processes have been identified. TgPhIL1, a Toxoplasma gondii protein that was previously identified through photosensitized labeling with 5-[(125)I] iodonapthaline-1-azide, associates with the IMC and/or underlying cytoskeleton and is concentrated at the apical end of the parasite. Orthologs of TgPhIL1 are found in other apicomplexans, but the function of this conserved protein family is unknown. As a first step towards determining the function of TgPhIL1 and its orthologs, we generated a T. gondii parasite line in which the single copy of TgPhIL1 was disrupted by homologous recombination. The TgPhIL1 knockout parasites have a distinctly different morphology than wild-type parasites, and normal shape is restored in the knockout background after complementation with the wild-type allele. The knockout parasites are outcompeted in culture by parasites expressing functional TgPhIL1, and they generate a reduced parasite load in the spleen and liver of infected mice. These findings demonstrate a role for TgPhIL1 in the morphology, growth and fitness of T. gondii tachyzoites.
Conflict of interest statement
Figures





References
-
- Mital J, Ward GE. Current and Emerging Approaches to Studying Invasion in Apicomplexan Parasites. Subcell Biochem. 2007;47:1–32. - PubMed
-
- Mann T, Beckers C. Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:257–268. - PubMed
-
- Dubremetz J-F, Torpier G, Maurois P, Prensier G, Sinden R. [Structure of the sporozoite pellicule of Plasmodium yoelii: a cryofracture study]. C. R. Acad Sci Paris. 1979;288:623–626.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

