Assessment of daily profiles of ADHD and ODD symptoms, and symptomatology related to ADHD medication, by parent and teacher ratings
- PMID: 21901413
- PMCID: PMC3180560
- DOI: 10.1007/s00787-011-0206-0
Assessment of daily profiles of ADHD and ODD symptoms, and symptomatology related to ADHD medication, by parent and teacher ratings
Abstract
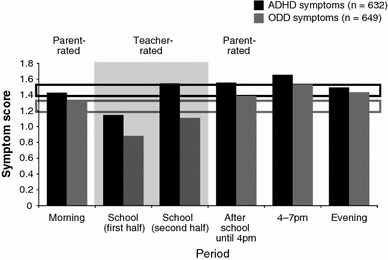
DAYAS is a new two-part rating scale that assesses: (1) ADHD and ODD symptoms (externalising symptom ratings) and (2) symptomatology potentially related to ADHD medication (potentially medication-related symptoms) in real-world settings at different time periods throughout a normal school day. Data from a proof-of-concept study and two observational trials (Medikinet(®) retard [methylphenidate] and the Equasym XL(®) [methylphenidate] OBSEER study) evaluated: (1) validity of weekly externalising symptom ratings using DAYAS, in place of daily ratings; (2) reliability and internal consistency of DAYAS ratings for externalising symptoms and potentially medication-related symptoms; and (3) convergent and divergent validity of the externalising symptom ratings with existing validated scales. From the proof-of-concept study, daily scores by period of day and during the whole day correlated strongly with equivalent weekly scores (r = 0.83-0.92). Internal consistency of externalising symptom rating scales calculated from pooled data were acceptable or good by period of day (Cronbach's alpha = 0.68-0.90) and very high for whole day scores (Cronbach's alpha = 0.88-0.95). Internal consistency of the rating scale for potentially medication-related symptoms was also good for both teacher and parent ratings. From OBSEER data, correlations between FBB-ADHD total symptom scores and ratings on both parent and teacher versions of DAYAS were high (r = 0.73 and r = 0.84, respectively). Correlations between DAYAS and SDQ were highest for the SDQ subscales hyperactivity and conduct problems and substantially lower for pro-social behaviour, peers and emotional problems. The DAYAS rating scale had good internal consistency, and DAYAS scores correlated well with existing validated scales and the SDQ subscales hyperactivity and conduct problems. Weekly DAYAS scores (whole day and by period of day) could be considered a suitable replacement for daily assessment scores.
Figures
References
-
- Bruhl B, Döpfner M, Lehmkuhl G (2000) Der Fremdbeurteilungsbogen für hyperkinetische Störungen (FBB-HKS)—Prävalenz hyperkinetischer Störrungen im Elternurteil und psychometrische Kriterien. Kindheit und Entwicklung 9:116–126
-
- Conners CK. Conners’ rating scales—revised. Multi-Health Systems Inc, New York: Technical manual; 1997.
-
- CTADD (2010) Guide to an outpatient medication assessment. http://ccf.buffalo.edu/pdf/MedAsses.pdf. Date Accessed 22-12-2010
-
- Döpfner M, Lehmkuhl G, Steinhausen HC. Diagnostische Verfahren, Kinder-Diagnostik-System (KIDS), Band 1: Aufmerksamkeitsdefizit- und Hyperaktivitätsstörungen (ADHS) Göttingen: Hogrefe; 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

