An observational study of once-daily modified-release methylphenidate in ADHD: the effect of previous treatment on ADHD symptoms, other externalising symptoms and quality-of-life outcomes
- PMID: 21901414
- PMCID: PMC3098980
- DOI: 10.1007/s00787-011-0205-1
An observational study of once-daily modified-release methylphenidate in ADHD: the effect of previous treatment on ADHD symptoms, other externalising symptoms and quality-of-life outcomes
Abstract
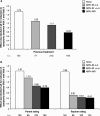
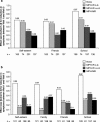
Methylphenidate (MPH) is the most commonly prescribed stimulant for children with ADHD. Data on the effects of different MPH formulations in real-life settings are scarce, and the role of previous therapy on treatment outcome when switching medications has not been well studied. OBSEER was an observational study designed to evaluate the effectiveness and safety of Equasym XL(®) in routine care. This study assessed whether the improvements reported with Equasym XL(®) are influenced by the degree of symptom control achieved with the previous medication. Patients enrolled in OBSEER were stratified by prior treatment (none, MPH-immediate release [IR] once daily [o.d.] [MPH-IR o.d.], MPH-IR repeated [MPH-IR >o.d.] and MPH-MR [modified release] excluding Equasym XL(®)), and changes in ADHD and other externalising symptoms (CGI-S, FBB-ADHD and DAYAS) and quality of life (QoL, KINDL) were evaluated during treatment with Equasym XL(®). A total of 782 patients were analysed. Significant group-by-time interactions were found for all symptom variables analysed, indicating that effects varied by previous medication. For CGI-S and FBB-ADHD total scores, the greatest reductions in ADHD symptoms were observed in the treatment-naïve subgroup, followed (in order) by MPH-IR o.d., MPH-IR >o.d. and MPH-MR. A similar profile was seen for DAYAS ratings for all periods of the day except the evening, when there were no significant differences between subgroups. Similarly, the treatment-naïve and MPH-IR o.d. subgroups showed the greatest improvements in KINDL ratings. Although effects were greatest for treatment-naïve patients, improvements were also observed in the prior treatment subgroups for symptoms and QoL. This suggests that a change to Equasym XL(®) may be beneficial in patients with suboptimal effects on prior medication.
Figures
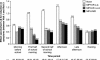
Similar articles
-
An observational study of once-daily modified-release methylphenidate in ADHD: quality of life, satisfaction with treatment and adherence.Eur Child Adolesc Psychiatry. 2011 Oct;20 Suppl 2(Suppl 2):S257-65. doi: 10.1007/s00787-011-0203-3. Eur Child Adolesc Psychiatry. 2011. PMID: 21901416 Free PMC article.
-
An observational study of once-daily modified-release methylphenidate in ADHD: effectiveness on symptoms and impairment, and safety.Eur Child Adolesc Psychiatry. 2011 Oct;20 Suppl 2(Suppl 2):S243-55. doi: 10.1007/s00787-011-0202-4. Eur Child Adolesc Psychiatry. 2011. PMID: 21901417 Free PMC article.
-
Assessment of daily profiles of ADHD and ODD symptoms, and symptomatology related to ADHD medication, by parent and teacher ratings.Eur Child Adolesc Psychiatry. 2011 Oct;20 Suppl 2(Suppl 2):S289-96. doi: 10.1007/s00787-011-0206-0. Eur Child Adolesc Psychiatry. 2011. PMID: 21901413 Free PMC article.
-
A review of OROS methylphenidate (Concerta(®)) in the treatment of attention-deficit/hyperactivity disorder.CNS Drugs. 2014 Nov;28(11):1005-33. doi: 10.1007/s40263-014-0175-1. CNS Drugs. 2014. PMID: 25120227 Review.
-
Drug Regimen Individualization for Attention-Deficit/Hyperactivity Disorder: Guidance for Methylphenidate and Dexmethylphenidate Formulations.Pharmacotherapy. 2019 Jun;39(6):677-688. doi: 10.1002/phar.2190. Epub 2018 Nov 23. Pharmacotherapy. 2019. PMID: 30351459 Review.
Cited by
-
A comparative study on the neurophysiological mechanisms underlying effects of methylphenidate and neurofeedback on inhibitory control in attention deficit hyperactivity disorder.Neuroimage Clin. 2018;20:1191-1203. doi: 10.1016/j.nicl.2018.10.027. Epub 2018 Oct 25. Neuroimage Clin. 2018. PMID: 30390574 Free PMC article.
-
Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents - assessment of adverse events in non-randomised studies.Cochrane Database Syst Rev. 2018 May 9;5(5):CD012069. doi: 10.1002/14651858.CD012069.pub2. Cochrane Database Syst Rev. 2018. PMID: 29744873 Free PMC article.
-
An observational study of response heterogeneity in children with attention deficit hyperactivity disorder following treatment switch to modified-release methylphenidate.BMC Psychiatry. 2013 Sep 3;13:219. doi: 10.1186/1471-244X-13-219. BMC Psychiatry. 2013. PMID: 24004962 Free PMC article.
-
Editorial: Observational studies in ADHD: the effects of switching to modified-release methylphenidate preparations on clinical outcomes and adherence.Eur Child Adolesc Psychiatry. 2011 Oct;20 Suppl 2(Suppl 2):S235-42. doi: 10.1007/s00787-011-0201-5. Eur Child Adolesc Psychiatry. 2011. PMID: 21901418 Free PMC article.
-
Effectiveness and safety of a long-acting, once-daily, two-phase release formulation of methylphenidate (Ritalin ® LA) in school children under daily practice conditions.Atten Defic Hyperact Disord. 2015 Jun;7(2):157-64. doi: 10.1007/s12402-014-0154-x. Epub 2014 Oct 28. Atten Defic Hyperact Disord. 2015. PMID: 25346231 Free PMC article.
References
-
- American Psychiatric Association (2000) Attention-deficit and disruptive behavior disorders. Attention-deficit/hyperactivity disorder. Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Arlington, pp 85–103
-
- Arnold LE, Bozzolo DR, Hodgkins P, McKay M, Beckett-Thurman L, Greenbaum M, Bukstein O, Patel A. Switching from oral extended-release methylphenidate to the methylphenidate transdermal system: continued attention-deficit/hyperactivity disorder symptom control and tolerability after abrupt conversion. Curr Med Res Opin. 2010;26:129–137. doi: 10.1185/03007990903437412. - DOI - PMC - PubMed
-
- Banaschewski T, Coghill D, Santosh P, Zuddas A, Asherson P, Buitelaar J, Danckaerts M, Döpfner M, Faraone SV, Rothenberger A, Sergeant J, Steinhausen HC, Sonuga-Barke EJ, Taylor E. Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry. 2006;15:476–495. doi: 10.1007/s00787-006-0549-0. - DOI - PubMed
-
- Bruhl B, Döpfner M, Lehmkuhl G (2000) Der Fremdbeurteilungsbogen für hyperkinetische Störungen (FBB-HKS)—Prävalenz hyperkinetischer Störrungen im Elternurteil und psychometrische Kriterien. Kindheit Entwicklung 9 S:116–126
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

