An observational study of once-daily modified-release methylphenidate in ADHD: quality of life, satisfaction with treatment and adherence
- PMID: 21901416
- PMCID: PMC3180635
- DOI: 10.1007/s00787-011-0203-3
An observational study of once-daily modified-release methylphenidate in ADHD: quality of life, satisfaction with treatment and adherence
Abstract
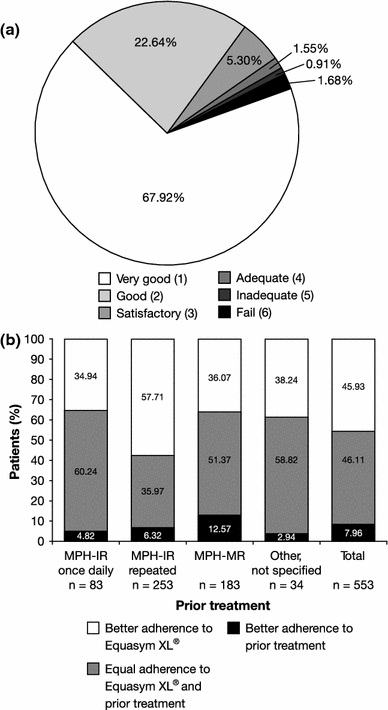
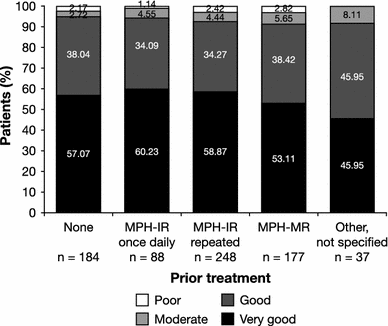
Attention deficit hyperactivity disorder (ADHD) impacts significantly on the quality of life (QoL) of patients and their families. Choice of therapy is increasingly influenced by treatment satisfaction and patient preference, with once-daily modified-release methylphenidate (MPH-MR) formulations offering clear benefits compared with immediate-release (IR) dosage forms. The effects of MPH-MR on QoL in ADHD have not been widely investigated and need more clarity in practice. The open-label OBSEER study evaluated the effectiveness and tolerability of Equasym XL(®), a MPH-MR formulation, in routine practice. Children and adolescents (aged 6-17 years) with ADHD and attending school were included if Equasym XL(®) treatment was planned by the treating physician. Physicians, parents and patients completed questionnaires assessing QoL (KINDL; parent, child or adolescent versions), satisfaction with medication, adherence and treatment tolerability at baseline (Visit 1), 1-3 weeks (Visit 2) and 6-12 weeks (Visit 3) over a maximum 3-month observation period. Data from 822 consecutively referred patients were analysed. QoL and medication satisfaction increased from Visit 1 to Visit 3, with both patients and parents rating therapy with Equasym XL(®) as better than previous drug therapy. KINDL total score effect sizes were 0.67 (parents' ratings), 0.52 (children's ratings) and 0.51 (adolescents' ratings; all p < 0.001). All KINDL subscores also increased: both parents and patients had the greatest improvement for school. Adherence to Equasym XL(®) was frequently rated as superior to prior treatment, particularly compared with MPH-IR repeated dosing. Treatment was generally well tolerated; approximately 3% of the patients discontinued treatment due to adverse events. Equasym XL(®) improved QoL compared with prior therapy, and resulted in good medication satisfaction and adherence in drug-naïve and previously treated patients.
Figures

Similar articles
-
An observational study of once-daily modified-release methylphenidate in ADHD: the effect of previous treatment on ADHD symptoms, other externalising symptoms and quality-of-life outcomes.Eur Child Adolesc Psychiatry. 2011 Oct;20 Suppl 2(Suppl 2):S277-88. doi: 10.1007/s00787-011-0205-1. Eur Child Adolesc Psychiatry. 2011. PMID: 21901414 Free PMC article.
-
An observational study of once-daily modified-release methylphenidate in ADHD: effectiveness on symptoms and impairment, and safety.Eur Child Adolesc Psychiatry. 2011 Oct;20 Suppl 2(Suppl 2):S243-55. doi: 10.1007/s00787-011-0202-4. Eur Child Adolesc Psychiatry. 2011. PMID: 21901417 Free PMC article.
-
Relationship between quality of life and psychopathological profile: data from an observational study in children with ADHD.Eur Child Adolesc Psychiatry. 2011 Oct;20 Suppl 2(Suppl 2):S267-75. doi: 10.1007/s00787-011-0204-2. Eur Child Adolesc Psychiatry. 2011. PMID: 21901415 Free PMC article.
-
A review of OROS methylphenidate (Concerta(®)) in the treatment of attention-deficit/hyperactivity disorder.CNS Drugs. 2014 Nov;28(11):1005-33. doi: 10.1007/s40263-014-0175-1. CNS Drugs. 2014. PMID: 25120227 Review.
-
Spotlight on methylphenidate controlled-delivery capsules (Equasym XL, Metadate CD) in the treatment of children and adolescents with attention-deficit hyperactivity disorder.CNS Drugs. 2007;21(2):173-5. doi: 10.2165/00023210-200721020-00007. CNS Drugs. 2007. PMID: 17284098 Review.
Cited by
-
Editorial: Observational studies in ADHD: the effects of switching to modified-release methylphenidate preparations on clinical outcomes and adherence.Eur Child Adolesc Psychiatry. 2011 Oct;20 Suppl 2(Suppl 2):S235-42. doi: 10.1007/s00787-011-0201-5. Eur Child Adolesc Psychiatry. 2011. PMID: 21901418 Free PMC article.
-
Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents - assessment of adverse events in non-randomised studies.Cochrane Database Syst Rev. 2018 May 9;5(5):CD012069. doi: 10.1002/14651858.CD012069.pub2. Cochrane Database Syst Rev. 2018. PMID: 29744873 Free PMC article.
-
Psychopharmacological treatment in children: always keeping an eye on adherence and ethics.Eur Child Adolesc Psychiatry. 2013 Aug;22(8):453-5. doi: 10.1007/s00787-013-0445-3. Eur Child Adolesc Psychiatry. 2013. PMID: 23824471 No abstract available.
-
Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies.BMC Psychiatry. 2013 Sep 27;13:237. doi: 10.1186/1471-244X-13-237. BMC Psychiatry. 2013. PMID: 24074240 Free PMC article.
-
An observational study of once-daily modified-release methylphenidate in ADHD: the effect of previous treatment on ADHD symptoms, other externalising symptoms and quality-of-life outcomes.Eur Child Adolesc Psychiatry. 2011 Oct;20 Suppl 2(Suppl 2):S277-88. doi: 10.1007/s00787-011-0205-1. Eur Child Adolesc Psychiatry. 2011. PMID: 21901414 Free PMC article.
References
-
- American Psychiatric Association . Attention-deficit and disruptive behavior disorders. Attention-deficit/hyperactivity disorder. Diagnostic and statistical manual of mental disorders. Fourth. Arlington: American Psychiatric Association; 2000. pp. 85–103.
-
- Bukstein OG. Satisfaction with treatment for attention-deficit/hyperactivity disorder. Am J Manag Care. 2004;10:S107–S116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

