FOXP1, an estrogen-inducible transcription factor, modulates cell proliferation in breast cancer cells and 5-year recurrence-free survival of patients with tamoxifen-treated breast cancer
- PMID: 21901488
- PMCID: PMC10358050
- DOI: 10.1007/s12672-011-0082-6
FOXP1, an estrogen-inducible transcription factor, modulates cell proliferation in breast cancer cells and 5-year recurrence-free survival of patients with tamoxifen-treated breast cancer
Abstract
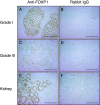
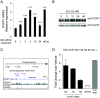
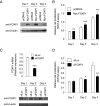
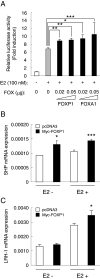
Breast cancer is primarily a hormone-dependent tumor that can be regulated by the status of steroid hormones, including estrogen and progesterone. Forkhead box P1 (FOXP1) is a member of the forkhead box transcription factor family and has been reported to be associated with various types of tumors. In the present study, we investigated the expression of FOXP1 in 133 human invasive breast cancers, obtained by core biopsy, by immunohistochemical analysis. Nuclear immunoreactivity of FOXP1 was detected in 89 cases (67%) and correlated positively with tumor grade and hormone receptor status, including estrogen receptor alpha (ERα) and progesterone receptor, and negatively with pathological tumor size. In ERα-positive MCF-7 breast cancer cells, we demonstrated that FOXP1 mRNA was upregulated by estrogen and increased ERα recruitment to ER binding sites identified by ChIP-on-chip analysis within the FOXP1 gene region. We also demonstrated that proliferation of MCF-7 cells was increased by exogenously transfected FOXP1 and decreased by FOXP1-specific siRNA. Furthermore, FOXP1 enhanced estrogen response element-driven transcription in MCF-7 cells. Finally, FOXP1 immunoreactivity was significantly elevated in relapse-free breast cancer patients treated with tamoxifen. These results suggest that FOXP1 plays an important role in proliferation of breast cancer cells by modulating estrogen signaling and that FOXP1 immunoreactivity could be associated with the estrogen dependency of clinical breast cancers, which may predict favorable prognosis in the patients treated with tamoxifen.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Association of double-positive FOXA1 and FOXP1 immunoreactivities with favorable prognosis of tamoxifen-treated breast cancer patients.Horm Cancer. 2012 Aug;3(4):147-59. doi: 10.1007/s12672-012-0111-0. Epub 2012 Apr 3. Horm Cancer. 2012. PMID: 22476979 Free PMC article.
-
Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptor-beta in primary invasive breast carcinomas.Breast Cancer Res Treat. 2008 Oct;111(3):453-9. doi: 10.1007/s10549-007-9812-4. Epub 2007 Nov 17. Breast Cancer Res Treat. 2008. PMID: 18026833
-
FOXP1 and estrogen signaling in breast cancer.Vitam Horm. 2013;93:203-12. doi: 10.1016/B978-0-12-416673-8.00006-X. Vitam Horm. 2013. PMID: 23810008 Review.
-
Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor alpha and improved survival in primary human breast carcinomas.Clin Cancer Res. 2004 May 15;10(10):3521-7. doi: 10.1158/1078-0432.CCR-03-0461. Clin Cancer Res. 2004. PMID: 15161711
-
The nature of tamoxifen action in the control of female breast cancer.In Vivo. 2001 Jul-Aug;15(4):319-25. In Vivo. 2001. PMID: 11695224 Review.
Cited by
-
Association of double-positive FOXA1 and FOXP1 immunoreactivities with favorable prognosis of tamoxifen-treated breast cancer patients.Horm Cancer. 2012 Aug;3(4):147-59. doi: 10.1007/s12672-012-0111-0. Epub 2012 Apr 3. Horm Cancer. 2012. PMID: 22476979 Free PMC article.
-
Regulation of aromatase in cancer.Mol Cell Biochem. 2021 Jun;476(6):2449-2464. doi: 10.1007/s11010-021-04099-0. Epub 2021 Feb 18. Mol Cell Biochem. 2021. PMID: 33599895 Review.
-
FOXP1 forkhead transcription factor is associated with the pathogenesis of endometrial cancer.Heliyon. 2016 May 27;2(5):e00116. doi: 10.1016/j.heliyon.2016.e00116. eCollection 2016 May. Heliyon. 2016. PMID: 27441287 Free PMC article.
-
The untold stories of the speech gene, the FOXP2 cancer gene.Genes Cancer. 2018 Jan;9(1-2):11-38. doi: 10.18632/genesandcancer.169. Genes Cancer. 2018. PMID: 29725501 Free PMC article. Review.
-
MiR-206 is down-regulated and suppresses cell proliferation by targeting FOXP1 in brain gliomas.Int J Clin Exp Pathol. 2018 Jul 1;11(7):3405-3415. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31949718 Free PMC article.
References
-
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistelinger TR, Fox EA, Silver PA, Brown M. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

