Mono- and dinuclear iron complexes of bis(1-methylimidazol-2-yl)ketone (bik): structure, magnetic properties, and catalytic oxidation studies
- PMID: 21902227
- PMCID: PMC3221465
- DOI: 10.1021/ic200332y
Mono- and dinuclear iron complexes of bis(1-methylimidazol-2-yl)ketone (bik): structure, magnetic properties, and catalytic oxidation studies
Abstract
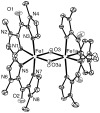
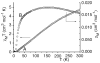
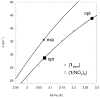
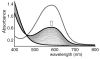
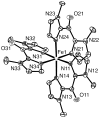
The newly synthesized dinuclear complex [Fe(III)(2)(μ-OH)(2)(bik)(4)](NO(3))(4) (1) (bik, bis(1-methylimidazol-2-yl)ketone) shows rather short Fe···Fe (3.0723(6) Å) and Fe-O distances (1.941(2)/1.949(2) Å) compared to other unsupported Fe(III)(2)(μ-OH)(2) complexes. The bridging hydroxide groups of 1 are strongly hydrogen-bonded to a nitrate anion. The (57)Fe isomer shift (δ = 0.45 mm s(-1)) and quadrupole splitting (ΔE(Q) = 0.26 mm s(-1)) obtained from Mössbauer spectroscopy are consistent with the presence of two identical high-spin iron(III) sites. Variable-temperature magnetic susceptibility studies revealed antiferromagnetic exchange (J = 35.9 cm(-1) and H = JS(1)·S(2)) of the metal ions. The optimized DFT geometry of the cation of 1 in the gas phase agrees well with the crystal structure, but both the Fe···Fe and Fe-OH distances are overestimated (3.281 and 2.034 Å, respectively). The agreement in these parameters improves dramatically (3.074 and 1.966 Å) when the hydrogen-bonded nitrate groups are included, reducing the value calculated for J by 35%. Spontaneous reduction of 1 was observed in methanol, yielding a blue [Fe(II)(bik)(3)](2+) species. Variable-temperature magnetic susceptibility measurements of [Fe(II)(bik)(3)](OTf)(2) (2) revealed spin-crossover behavior. Thermal hysteresis was observed with 2, due to a loss of cocrystallized solvent molecules, as monitored by thermogravimetric analysis. The hysteresis disappears once the solvent is fully depleted by thermal cycling. [Fe(II)(bik)(3)](OTf)(2) (2) catalyzes the oxidation of alkanes with t-BuOOH. High selectivity for tertiary C-H bond oxidation was observed with adamantane (3°/2° value of 29.6); low alcohol/ketone ratios in cyclohexane and ethylbenzene oxidation, a strong dependence of total turnover number on the presence of O(2), and a low retention of configuration in cis-1,2-dimethylcyclohexane oxidation were observed. Stereoselective oxidation of olefins with dihydrogen peroxide yielding epoxides was observed under both limiting oxidant and substrate conditions.
Figures
Similar articles
-
Accessibility and selective stabilization of the principal spin states of iron by pyridyl versus phenolic ketimines: modulation of the 6A1 ↔ 2T2 ground-state transformation of the [FeN4O2]+ chromophore.Inorg Chem. 2012 Aug 6;51(15):8241-53. doi: 10.1021/ic300732r. Epub 2012 Jul 18. Inorg Chem. 2012. PMID: 22808945
-
Synthesis and characterization of homo- and heterodinuclear M(II)-M'(III) (M(II) = Mn or Fe, M'(III) = Fe or Co) mixed-valence supramolecular pseudo-dimers. The effect of hydrogen bonding on spin state selection of M(II).Dalton Trans. 2011 Jan 7;40(1):181-94. doi: 10.1039/c0dt01098g. Epub 2010 Nov 19. Dalton Trans. 2011. PMID: 21103467
-
Thermally-Induced Spin Crossover and LIESST Effect in the Neutral [FeII(Mebik)2(NCX)2] Complexes: Variable-Temperature Structural, Magnetic, and Optical Studies (X = S, Se; Mebik = bis(1-methylimidazol-2-yl)ketone).Front Chem. 2018 Aug 21;6:326. doi: 10.3389/fchem.2018.00326. eCollection 2018. Front Chem. 2018. PMID: 30186827 Free PMC article.
-
Characterization of three members of the electron-transfer series [Fe(pda)2]n (n=2-, 1-, 0) by spectroscopy and density functional theoretical calculations [pda=redox non-innocent derivatives of N,N'-bis(pentafluorophenyl)-o-phenylenediamide(2-, 1.-, 0)].Chemistry. 2008;14(25):7608-22. doi: 10.1002/chem.200800546. Chemistry. 2008. PMID: 18601237
-
A structural and Mössbauer study of complexes with Fe(2)(micro-O(H))(2) cores: stepwise oxidation from Fe(II)(micro-OH)(2)Fe(II) through Fe(II)(micro-OH)(2)Fe(III) to Fe(III)(micro-O)(micro-OH)Fe(III).Inorg Chem. 2004 May 17;43(10):3067-79. doi: 10.1021/ic030296k. Inorg Chem. 2004. PMID: 15132612
Cited by
-
Isolation and characterization of a dihydroxo-bridged iron(III,III)(μ-OH)2 diamond core derived from dioxygen.Inorg Chem. 2013 Dec 2;52(23):13325-31. doi: 10.1021/ic4010906. Epub 2013 Nov 14. Inorg Chem. 2013. PMID: 24229319 Free PMC article.
-
Quantitative Assessment of rPM6 for Fluorine- and Chlorine-Containing Metal Complexes: Comparison with Experimental, First-Principles, and Other Semiempirical Results.Molecules. 2018 Dec 15;23(12):3332. doi: 10.3390/molecules23123332. Molecules. 2018. PMID: 30558286 Free PMC article.
-
Analysis of the Puzzling Exchange-Coupling Constants in a Series of Heterobimetallic Complexes.Inorg Chem. 2019 Jul 15;58(14):9150-9160. doi: 10.1021/acs.inorgchem.9b00757. Epub 2019 Jun 26. Inorg Chem. 2019. PMID: 31241914 Free PMC article.
References
-
- Tanase S, Bouwman E. Adv Inorg Chem. 2006;58:29.
-
- Bruijnincx PCA, Van Koten G, Klein Gebbink RJM. Chem Soc Rev. 2008;37:2716. - PubMed
-
- Costas M, Mehn MP, Jensen MP, Que L., Jr Chem Rev. 2004;104:939. - PubMed
-
- Solomon EI, Brunold TC, Davis MI, Kernsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J. Chem Rev. 2000;100:235. - PubMed
-
- Tshuva EY, Lippard SJ. Chem Rev. 2004;104:987. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

