Biosynthesis of cholesterol and other sterols
- PMID: 21902244
- PMCID: PMC3191736
- DOI: 10.1021/cr200021m
Biosynthesis of cholesterol and other sterols
Figures




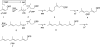

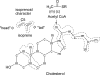



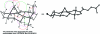
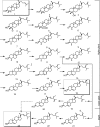



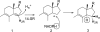

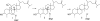

References
-
- Myant N. B.The Biology of Cholesterol and Related Steroids; William Heinemann Medical Books Ltd.: London, 1981; p 1.
-
- Nes W. R.; McKean M. L.. Biochemistry of Steroids and Other Isopentenoids; University Park Press: Baltimore, MD, 1977; p 1.
-
- Brown M. S.; Goldstein. J. L. Science 1986, 232, 34. - PubMed
- http://nobelprize.org/.
-
- Gibbons G. F. Lipids 2002, 37, 1153. - PubMed
-
- Popjak G. In Lipids: Chemistry, Biochemistry and Nutrition; Mead J. F., Alfin-Slater R. B., Howton D. R., Popjak. G., Eds.; Plenum Press: New York, 1986; p 295.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

