Impact of intravenous naltrexone on intravenous morphine-induced high, drug liking, and euphoric effects in experienced, nondependent male opioid users
- PMID: 21902287
- PMCID: PMC3586102
- DOI: 10.2165/11593390-000000000-00000
Impact of intravenous naltrexone on intravenous morphine-induced high, drug liking, and euphoric effects in experienced, nondependent male opioid users
Abstract
Background: Opioid analgesics can be abused by crushing followed by solubilization and intravenous injection to attain rapid absorption. Morphine sulfate and naltrexone hydrochloride extended release capsules (EMBEDA, MS-sNT), indicated for management of chronic, moderate to severe pain, contain pellets of morphine sulfate with a sequestered naltrexone core. Should product tampering by crushing occur, the sequestered naltrexone is intended for release to reduce morphine-induced subjective effects.
Objective: This study compared self-reports of high, euphoria, and drug-liking effects of intravenous morphine alone versus intravenous morphine combined with naltrexone in a clinical simulation of intravenous abuse of crushed MS-sNT.
Methods: This single-center, randomized, double-blind, crossover study characterized subjective effects of naltrexone administered intravenously at the same ratio to morphine present in MS-sNT. Subjects were male and had used prescription opioids five or more times within the previous 12 months to get 'high' but were not physically dependent on opioids. The primary outcome was the response to the Drug Effects Questionnaire (DEQ) question #5, "How high are you now?" (100 mm Visual Analog Scale [VAS]). The secondary outcome was the response to a Cole/Addiction Research Center Inventory (ARCI) Stimulation-Euphoria modified scale. Additional outcomes included response to VAS drug liking, the remaining DEQ questions, and pupillometry.
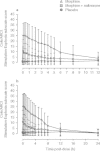
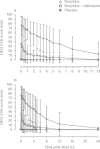
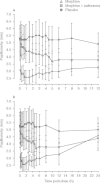
Results: Administration of intravenous naltrexone following intravenous morphine diminished mean high (29.8 vs 85.2 mm), Cole/ARCI Stimulation-Euphoria (13.7 vs 27.8 mm), and drug-liking (38.9 vs 81.4 mm) scores (all p < 0.0001) compared with intravenous morphine alone. No serious adverse events occurred as a result of the tested ratio of naltrexone to morphine.
Conclusions: Results in this study population suggest that naltrexone added to morphine in the 4% ratio within MS-sNT mitigates the high, euphoria, and drug liking of morphine alone, potentially reducing the attractiveness for product tampering. Assessment of the true clinical significance of these findings will require further study.
Figures







References
-
- Woolf CJ, Hashmi M. Use and abuse of opioid analgesics: potential methods to prevent and deter non-medical consumption of prescription opioids. Curr Opin Investig Drugs. 2004;5(1):61–6. - PubMed
-
- National Institute on Drug Abuse (NIDA). Prescription medications, NIDA news [online]. Available from URL: http://www.nida.nih.gov/DrugPages/prescription.html [Accessed 2009 Apr 21]
-
- Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Health: national findings [online]. Office of Applied Studies, NSDUH Series H-36, HHS publication no. (SMA) 09-4434. Rockville (MD). Available from URL: http://oas.samhsa.gov [Accessed 2009 Oct 2]
-
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2006: national estimates of drug-related emergency department visits [online]. Office of Applied Studies, DAWN Series D-30, DHHS publication no. (SMA) 08-4339. Rockville (MD). Available from URL: http://dawninfo.samhsa.gov [Accessed 2010 Aug 1]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
