Three-dimensional cell grafting enhances the angiogenic efficacy of human umbilical vein endothelial cells
- PMID: 21902465
- PMCID: PMC3267972
- DOI: 10.1089/ten.TEA.2011.0193
Three-dimensional cell grafting enhances the angiogenic efficacy of human umbilical vein endothelial cells
Abstract
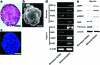
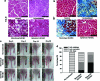
Despite the great potential of cell therapy for ischemic disease, poor cell survival after engraftment in ischemic tissue limits its efficacy. Here we tested a hypothesis that three-dimensionally grafted human umbilical vein endothelial cell (HUVEC) spheroids would exhibit improved angiogenic efficacy following transplantation into mouse ischemic limbs compared with HUVECs prepared by conventional two-dimensional monolayer culture. One day after surgical induction of hindlimb ischemia in athymic mice, HUVECs cultured in monolayer or HUVEC spheroids were transplanted intramuscularly into ischemic limbs. Four weeks after the treatment, in the spheroid HUVEC transplantation group, we observed increased hypoxia-inducible factor-1α expression, decreased apoptosis, and increased HUVEC survival in the ischemic tissue compared with the monolayer HUVEC transplantation group. Transplantation of HUVEC spheroids also resulted in enhanced and prolonged secretion of paracrine factors as well as enhanced expression of factors involved in the recruitment of circulating angiogenic progenitor cells. In summary, transplantation of HUVECs as spheroids enhanced cell survival, increased paracrine factor secretion, and showed a potential as a therapeutic method to treat ischemic tissue damages by promoting angiogenesis.
Figures





References
-
- Chekanov V. Akhtar M. Tchekanov G. Dangas G. Shehzad M.Z. Tio F. Adamian M. Colombo A. Roubin G. Leon M.B. Moses J.W. Kipshidze N.N. Transplantation of autologous endothelial cells induces angiogenesis. Pacing Clin Electrophysiol. 2003;26:496. - PubMed
-
- Kim E.J. Li R.K. Weisel R.D. Mickle D.A. Jia Z.Q. Tomita S. Sakai T. Yau T.M. Angiogenesis by endothelial cell transplantation. J Thorac Cardiovasc Surg. 2001;122:963. - PubMed
-
- Zhang M. Methot D. Poppa V. Fujio Y. Walsh K. Murry C.E. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907. - PubMed
-
- Nakamura Y. Yasuda T. Weisel R.D. Li R.K. Enhanced cell transplantation: preventing apoptosis increases cell survival and ventricular function. Am J Physiol Heart Circ Physiol. 2006;291:H939. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

