Novel epitope evoking CD138 antigen-specific cytotoxic T lymphocytes targeting multiple myeloma and other plasma cell disorders
- PMID: 21902685
- PMCID: PMC3782999
- DOI: 10.1111/j.1365-2141.2011.08850.x
Novel epitope evoking CD138 antigen-specific cytotoxic T lymphocytes targeting multiple myeloma and other plasma cell disorders
Erratum in
- Br J Haematol. 2011 Dec;155(5):642
Abstract
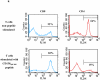
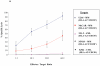
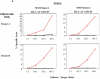
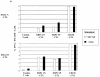
The development of an immunotherapeutic strategy targeting CD138 antigen could potentially represent a new treatment option for multiple myeloma (MM). This study evaluated the immune function of CD138 peptide-specific cytotoxic T lymphocytes (CTL), generated ex vivo using an HLA-A2-specific CD138 epitope against MM cells. A novel immunogenic HLA-A2-specific CD138(260-268) (GLVGLIFAV) peptide was identified from the full-length protein sequence of the CD138 antigen, which induced CTL specific to primary CD138(+) MM cells. The peptide-induced CD138-CTL contained a high percentage of CD8(+) activated/memory T cells with a low percentage of CD4(+) T cell and naive CD8(+) T cell subsets. The CTL displayed HLA-A2-restricted and CD138 antigen-specific cytotoxicity against MM cell lines. In addition, CD138-CTL demonstrated increased degranulation, proliferation and γ-interferon secretion to HLA-A2(+) /CD138(+) myeloma cells, but not HLA-A2(-) /CD138(+) or HLA-A2(+) /CD138(-) cells. The immune functional properties of the CD138-CTL were also demonstrated using primary HLA-A2(+) /CD138(+) cells isolated from myeloma patients. In conclusion, a novel immunogenic CD138(260-268) (GLVGLIFAV) peptide can induce antigen-specific CTL, which might be useful for the treatment of MM patients with peptide-based vaccine or cellular immunotherapy strategies.
2011 Blackwell Publishing Ltd.
Figures








References
-
- Bae J, Martinson JA, Klingemann HG. Identification of novel CD33 antigen-specific peptides for the generation of cytotoxic T lymphocytes against acute myeloid leukemia. Cellular Immunology. 2004;227:38–50. - PubMed
-
- Bae J, Martinson JA, Klingemann HG. Identification of CD19 and CD20 peptides for induction of antigen-specific CTLs against B-cell malignancies. Clinical Cancer Research. 2005;11:1629–1638. - PubMed
-
- Bagratuni T, Wu P, Gonzalez de Castro D, Davenport EL, Dickens NJ, Walker BA, Boyd K, Johnson DC, Gregory W, Morgan GJ, Davies FE. XBP1s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood. 2010;116:250–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

