The wandering Mirena: laparoscopic retrieval
- PMID: 21902959
- PMCID: PMC3134688
- DOI: 10.4293/108680811X13022985131732
The wandering Mirena: laparoscopic retrieval
Abstract
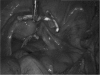
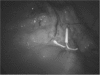
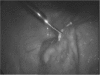
Levonorgestrel-containing intrauterine contraceptive devices, marketed as Mirena (Bayer HealthCare Pharmaceuticals, Inc. Australia) are widely used in contemporary gynecology, primarily as an effective method for contraception and for control of menstrual disorders like menorrhagia and dysmenorrhea. In this article, the authors report 2 cases of Mirena migration following intrauterine insertion by general practitioners (family physicians). In the first case, the contraceptive device had moved to the patient's right iliac fossa just anterior to the cecum and, in the second, within the peritoneal cavity close to the left leaf of the diaphragm. Both patients underwent uneventful laparoscopic retrieval of the devices.
Figures

References
-
- ESHRE Capri Workshop Group Intrauterine devices and intrauterine systems. Hum Reprod Update. 2008. May-Jun;14(3):197–208
-
- Wildemeersch D, Jansssens D, Andrade A. The Femilis LNG-IUS: Contraceptive performance- an interim analysis. Eur J Contracept Reprod Health Care. 2009. April;14(2):103–110 - PubMed
-
- Chen EC, Danis PG, Tweed E. Clinical inquiries. Menstrual disturbances in perimenopausal women: What's best? J Fam Pract. 2009. June;58(6):E3. - PubMed
-
- Bahamondes L, Petta CA, Fernandes A, Monteiro L. Use of Levonorgestrel-releasing intrauterine system in women with endometriosis, chronic pelvic pain and dysmenorrhea. Contraception. 2007. June;75(6 suppl):S123–S129 - PubMed
-
- Cameron S. Contraception and gynaecological care. Best Pract Res Clin Obstet Gynaecol. 2009. April;23(2):211–220 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
