Development of a method for the purification and culture of rodent astrocytes
- PMID: 21903074
- PMCID: PMC3172573
- DOI: 10.1016/j.neuron.2011.07.022
Development of a method for the purification and culture of rodent astrocytes
Abstract
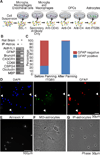
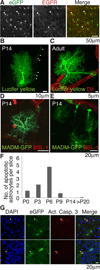
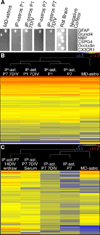
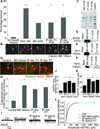
The inability to purify and culture astrocytes has long hindered studies of their function. Whereas astrocyte progenitor cells can be cultured from neonatal brain, culture of mature astrocytes from postnatal brain has not been possible. Here, we report a new method to prospectively purify astrocytes by immunopanning. These astrocytes undergo apoptosis in culture, but vascular cells and HBEGF promote their survival in serum-free culture. We found that some developing astrocytes normally undergo apoptosis in vivo and that the vast majority of astrocytes contact blood vessels, suggesting that astrocytes are matched to blood vessels by competing for vascular-derived trophic factors such as HBEGF. Compared to traditional astrocyte cultures, the gene profiles of the cultured purified postnatal astrocytes much more closely resemble those of in vivo astrocytes. Although these astrocytes strongly promote synapse formation and function, they do not secrete glutamate in response to stimulation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Banker GA. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980;209:809–810. - PubMed
-
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. - PubMed
-
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

