Down-regulation of the histone methyltransferase EZH2 contributes to the epigenetic programming of decidualizing human endometrial stromal cells
- PMID: 21903722
- PMCID: PMC3198959
- DOI: 10.1210/me.2011-1139
Down-regulation of the histone methyltransferase EZH2 contributes to the epigenetic programming of decidualizing human endometrial stromal cells
Abstract
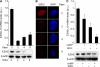
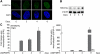
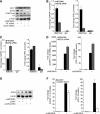
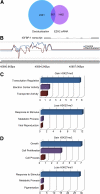
Differentiation of human endometrial stromal cells (HESC) into decidual cells represents a highly coordinated process essential for embryo implantation. We show that decidualizing HESC down-regulate the histone methyltransferase enhancer of Zeste homolog 2 (EZH2), resulting in declining levels of trimethylation of histone 3 on lysine 27 (H3K27me3) at the proximal promoters of key decidual marker genes PRL and IGFBP1. Loss of H3K27me3 was associated with a reciprocal enrichment in acetylation of the same lysine residue, indicating active remodeling from repressive to transcriptionally permissive chromatin. Chromatin immunoprecipitation coupled with DNA microarray analysis demonstrated that decidualization triggers genome-wide changes in H3K27me3 distribution that only partly overlap those observed upon EZH2 knockdown in undifferentiated HESC. Gene ontology revealed that gain of the repressive H3K27me3 mark in response to decidualization and upon EZH2 knockdown in undifferentiated cells was enriched at the promoter regions of genes involved in transcriptional regulation and growth/cell proliferation, respectively. However, loss of the H3K27me3 mark (indicating increased chromatin accessibility) in decidualizing cells and upon EZH2 knockdown occurred at selective loci enriched for genes functionally implicated in responses to stimulus. In agreement, EZH2 knockdown in undifferentiated HESC was sufficient to augment the induction of decidual marker genes in response to cyclic AMP and progesterone signaling. Thus, loss of EZH2-dependent methyltransferase activity in the endometrium is integral to the process of chromatin remodeling that enables the transition from a proliferative to a decidual phenotype in response to differentiation cues.
Figures


Similar articles
-
A central role for G9a and EZH2 in the epigenetic silencing of cyclooxygenase-2 in idiopathic pulmonary fibrosis.FASEB J. 2014 Jul;28(7):3183-96. doi: 10.1096/fj.13-241760. Epub 2014 Mar 20. FASEB J. 2014. PMID: 24652950 Free PMC article.
-
Bisphenol a affects endometrial stromal cells decidualization, involvement of epigenetic regulation.J Steroid Biochem Mol Biol. 2020 Jun;200:105640. doi: 10.1016/j.jsbmb.2020.105640. Epub 2020 Feb 19. J Steroid Biochem Mol Biol. 2020. PMID: 32087250
-
Epigenetic regulation of cell adhesion and communication by enhancer of zeste homolog 2 in human endothelial cells.Hypertension. 2012 Nov;60(5):1176-83. doi: 10.1161/HYPERTENSIONAHA.112.191098. Epub 2012 Sep 10. Hypertension. 2012. PMID: 22966008
-
EZH2 methyltransferase and H3K27 methylation in breast cancer.Int J Biol Sci. 2012;8(1):59-65. doi: 10.7150/ijbs.8.59. Epub 2011 Nov 18. Int J Biol Sci. 2012. PMID: 22211105 Free PMC article. Review.
-
Roles of the EZH2 histone methyltransferase in cancer epigenetics.Mutat Res. 2008 Dec 1;647(1-2):21-9. doi: 10.1016/j.mrfmmm.2008.07.010. Epub 2008 Aug 3. Mutat Res. 2008. PMID: 18723033 Review.
Cited by
-
Role of inflammation in benign gynecologic disorders: from pathogenesis to novel therapies†.Biol Reprod. 2021 Jul 2;105(1):7-31. doi: 10.1093/biolre/ioab054. Biol Reprod. 2021. PMID: 33739368 Free PMC article. Review.
-
Epigenetic Features in Uterine Leiomyosarcoma and Endometrial Stromal Sarcomas: An Overview of the Literature.Biomedicines. 2022 Oct 13;10(10):2567. doi: 10.3390/biomedicines10102567. Biomedicines. 2022. PMID: 36289829 Free PMC article. Review.
-
Understanding epigenetic regulation in the endometrium - lessons from mouse models with implantation defects.Epigenomics. 2025 Jun;17(8):541-554. doi: 10.1080/17501911.2025.2491298. Epub 2025 Apr 14. Epigenomics. 2025. PMID: 40228031 Review.
-
The clock protein period 2 synchronizes mitotic expansion and decidual transformation of human endometrial stromal cells.FASEB J. 2015 Apr;29(4):1603-14. doi: 10.1096/fj.14-267195. Epub 2015 Jan 8. FASEB J. 2015. PMID: 25573754 Free PMC article.
-
Potential mechanism of Luoshi Neiyi prescription in endometriosis based on serum pharmacochemistry and network pharmacology.Front Pharmacol. 2024 Jul 29;15:1395160. doi: 10.3389/fphar.2024.1395160. eCollection 2024. Front Pharmacol. 2024. PMID: 39135784 Free PMC article.
References
-
- Gellersen B, Brosens IA, Brosens JJ. 2007. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 - PubMed
-
- Wynn RM. 1974. Ultrastructural development of the human decidua. Am J Obstet Gynecol 118:652–670 - PubMed
-
- Brosens JJ, Gellersen B. 2006. Death or survival—progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol 36:389–398 - PubMed
-
- Brosens JJ, Hayashi N, White JO. 1999. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140:4809–4820 - PubMed
-
- Gellersen B, Brosens J. 2003. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 178:357–372 - PubMed

