A simple, multidimensional approach to high-throughput discovery of catalytic reactions
- PMID: 21903809
- PMCID: PMC3261636
- DOI: 10.1126/science.1207922
A simple, multidimensional approach to high-throughput discovery of catalytic reactions
Abstract
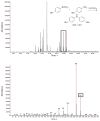
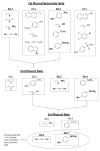
Transition metal complexes catalyze many important reactions that are employed in medicine, materials science, and energy production. Although high-throughput methods for the discovery of catalysts that would mirror related approaches for the discovery of medicinally active compounds have been the focus of much attention, these methods have not been sufficiently general or accessible to typical synthetic laboratories to be adopted widely. We report a method to evaluate a broad range of catalysts for potential coupling reactions with the use of simple laboratory equipment. Specifically, we screen an array of catalysts and ligands with a diverse mixture of substrates and then use mass spectrometry to identify reaction products that, by design, exceed the mass of any single substrate. With this method, we discovered a copper-catalyzed alkyne hydroamination and two nickel-catalyzed hydroarylation reactions, each of which displays excellent functional-group tolerance.
Figures
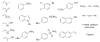
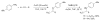
Comment in
-
Chemistry. High-throughput discovery of new chemical reactions.Science. 2011 Sep 9;333(6048):1387-8. doi: 10.1126/science.1210735. Science. 2011. PMID: 21903798 No abstract available.
References
-
- Eleuterio HS. J Mol Catal. 1991;65:55.
-
- Fischer K, Jonas K, Misbach P, Stabba R, Wilke G. Angew Chem Int Ed Engl. 1973;12:943.
-
- Stambuli JP, Hartwig JF. Curr Opin Chem Biol. 2003;7:420. - PubMed
-
- Wieland J, Breit B. Nat Chem. 2010;2:832. - PubMed
-
- Fagan PJ, Hauptman E, Shapiro R, Casalnuovo A. J Am Chem Soc. 2000;122:5043.

