Tolvaptan in autosomal dominant polycystic kidney disease: three years' experience
- PMID: 21903984
- PMCID: PMC3359559
- DOI: 10.2215/CJN.03530411
Tolvaptan in autosomal dominant polycystic kidney disease: three years' experience
Abstract
Background and objectives: Autosomal dominant polycystic kidney disease (ADPKD), a frequent cause of end-stage renal disease, has no cure. V2-specific vasopressin receptor antagonists delay disease progression in animal models.
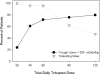
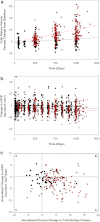
Design, setting, participants, and measurements: This is a prospectively designed analysis of annual total kidney volume (TKV) and thrice annual estimated GFR (eGFR) measurements, from two 3-year studies of tolvaptan in 63 ADPKD subjects randomly matched 1:2 to historical controls by gender, hypertension, age, and baseline TKV or eGFR. Prespecified end points were group differences in log-TKV (primary) and eGFR (secondary) slopes for month 36 completers, using linear mixed model (LMM) analysis. Sensitivity analyses of primary and secondary end points included LMM using all subject data and mixed model repeated measures (MMRM) of change from baseline at each year. Pearson correlation tested the association between log-TKV and eGFR changes.
Results: Fifty-one subjects (81%) completed 3 years of tolvaptan therapy; all experienced adverse events (AEs), with AEs accounting for six of 12 withdrawals. Baseline TKV (controls 1422, tolvaptan 1635 ml) and eGFR (both 62 ml/min per 1.73 m(2)) were similar. Control TKV increased 5.8% versus 1.7%/yr for tolvaptan (P < 0.001, estimated ratio of geometric mean 0.96 [95% confidence interval 0.95 to 0.97]). Corresponding annualized eGFR declined: -2.1 versus -0.71 ml/min per 1.73 m(2)/yr (P = 0.01, LMM group difference 1.1 ml/min per 1.73 m(2)/yr [95% confidence interval 0.24 to 1.9]). Sensitivity analyses including withdrawn subjects were similar, whereas MMRM analyses were significant at each year for TKV and nonsignificant for eGFR. Increasing TKV correlated with decreasing eGFR (r = -0.21, P < 0.01).
Conclusion: ADPKD cyst growth progresses more slowly with tolvaptan than in historical controls, but AEs are common.
Trial registration: ClinicalTrials.gov NCT00413777 NCT00841568.
Figures

References
-
- Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477–1485, 2008 - PubMed
-
- Gabow P: Autosomal dominant polycystic kidney disease. N Engl J Med 329: 323–342, 1993 - PubMed
-
- Dalgaard OZ: Bilateral polycystic disease of the kidneys: A follow-up of two hundred and eighty-four patients and their families. Acta Med Scand 328: 1–255, 1957 - PubMed
-
- Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 - PubMed
-
- Bajwa ZH, Gupta S, Warfield CA, Steinman TI: Pain management in polycystic kidney disease. Kidney Int 60: 1631–1644, 2001 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

