Dark progression reveals slow timescales for radiation damage between T = 180 and 240 K
- PMID: 21904032
- PMCID: PMC3169314
- DOI: 10.1107/S0907444911027600
Dark progression reveals slow timescales for radiation damage between T = 180 and 240 K
Abstract
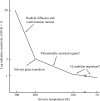
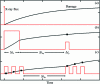
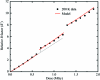
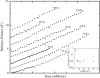
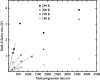
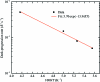
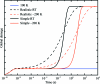
Can radiation damage to protein crystals be `outrun' by collecting a structural data set before damage is manifested? Recent experiments using ultra-intense pulses from a free-electron laser show that the answer is yes. Here, evidence is presented that significant reductions in global damage at temperatures above 200 K may be possible using conventional X-ray sources and current or soon-to-be available detectors. Specifically, `dark progression' (an increase in damage with time after the X-rays have been turned off) was observed at temperatures between 180 and 240 K and on timescales from 200 to 1200 s. This allowed estimation of the temperature-dependent timescale for damage. The rate of dark progression is consistent with an Arrhenius law with an activation energy of 14 kJ mol(-1). This is comparable to the activation energy for the solvent-coupled diffusive damage processes responsible for the rapid increase in radiation sensitivity as crystals are warmed above the glass transition near 200 K. Analysis suggests that at T = 300 K data-collection times of the order of 1 s (and longer at lower temperatures) may allow significant reductions in global radiation damage, facilitating structure solution on crystals with liquid solvent. No dark progression was observed below T = 180 K, indicating that no important damage process is slowed through this timescale window in this temperature range.
Figures
References
-
- Anbar, M. & Hart, E. J. (1967). J. Phys. Chem. 71, 3700–3702.
-
- Barker, A. I., Southworth-Davies, R. J., Paithankar, K. S., Carmichael, I. & Garman, E. F. (2009). J. Synchrotron Rad. 16, 205–216. - PubMed
-
- Benkovic, S. J. & Hammes-Schiffer, S. (2006). Science, 312, 208–209. - PubMed
-
- Blake, C. & Phillips, D. C. (1962). Proceedings of the Symposium on the Biological Effects of Ionizing Radiation at the Molecular Level, pp. 183–191. Vienna: International Atomic Energy Agency.
-
- Blundell, T. & Johnson, L. N. (1976). Protein Crystallography. London: Academic Press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

