Catalytic Addition of Simple Alkenes to Carbonyl Compounds Using Group 10 Metals
- PMID: 21904421
- PMCID: PMC3167217
- DOI: 10.1055/s-0029-1217747
Catalytic Addition of Simple Alkenes to Carbonyl Compounds Using Group 10 Metals
Abstract
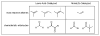
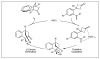
Recent advances using nickel complexes in the activation of unactivated monosubstituted olefins for catalytic intermolecular carbon-carbon bond-forming reactions with carbonyl compounds, such as simple aldehydes, isocyanates, and conjugated aldehydes and ketones, are discussed. In these reactions, the olefins function as vinyl- and allylmetal equivalents, providing a new strategy for organic synthesis. Current limitations and the outlook for this new strategy are also discussed.
Figures








References
-
- Lappin GR, Sauer JD, editors. Alpha Olefins Applications Handbook. M. Dekker; New York: 1989.
-
- Blom R, editor. Organometallic Catalysts and Olefin Polymerization. Springer; New York: 2001.
- Guest, Gladysz JA, editors. Chem Rev. 4 Vol. 100. 2000. Frontiers in Metal-Catalyzed Polymerization. - PubMed
-
- Tsuji J. Palladium Reagents and Catalysts: Innovations in Organic Synthesis. John Wiley & Sons; New York: 1995.
-
-
Ojima I, editor. Catalytic Asymmetric Synthesis. Wiley-VCH; New York: 2000. Recent reviews on epoxidation: Shi Y. Acc Chem Res. 2004;37:488.Yang D. Acc Chem Res. 2004;37:497.Xia QH, Ge HQ, Ye CP, Liu ZM, Su KX. Chem Rev. 2005;105:1603.Reviews on dihydroxylation: Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem Rev. 1994;94:2483.Bolm C, Hildebrand JP, Muniz K. In: Recent Advances in Asymmetric Dihydroxylation and Aminohydroxylation in Catalytic Asymmetric Synthesis. 2. Ojima I, editor. Wiley-VCH; Weinheim: 2000. p. 399.Sundermeier U, Döbler C, Beller M. In: Modern Oxidation Methods. Bäckvall J-E, editor. Wiley-VCH; Weinheim: 2004. pp. 1–20.Kobayashi S, Sugiura M. Adv Synth Catal. 2006;348:1496.
-
-
-
Grubbs RH, editor. Handbook of Metathesis. John Wiley & Sons; New York: 2003. Reviews: Grubbs RH. Tetrahedron. 2004;60:7117.Nicolaou KC, Bulger PG, Sarlah D. Angew Chem Int Ed. 2005;44:4490.
-
Grants and funding
LinkOut - more resources
Full Text Sources
