Improving antigenicity of the recombinant hepatitis C virus core protein via random mutagenesis
- PMID: 21904443
- PMCID: PMC3166572
- DOI: 10.1155/2011/359042
Improving antigenicity of the recombinant hepatitis C virus core protein via random mutagenesis
Abstract
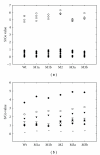
In order to enhance the sensitivity of diagnosis, a recombinant clone containing domain I of HCV core (amino acid residues 1 to 123) was subjected to random mutagenesis. Five mutants with higher sensitivity were obtained by colony screening of 616 mutants using reverse ELISA. Sequence analysis of these mutants revealed alterations focusing on W(84), P(95), P(110), or V(129). The inclusion bodies of these recombinant proteins overexpressed in E. coli BL21(DE3) were subsequently dissolved using 6 M urea and then refolded by stepwise dialysis. Compared to the unfolded wild-type antigen, the refolded M3b antigen (W(84)S, P(110)S and V(129)L) exhibited an increase of 66% antigenicity with binding capacity of 0.96 and affinity of 113 μM(-1). Moreover, the 33% decrease of the production demand suggests that M3b is a potential substitute for anti-HCV antibody detection.
Figures

References
-
- Mori Y, Moriishi K, Matsuura Y. Hepatitis C virus core protein: its coordinate roles with PA28γ in metabolic abnormality and carcinogenicity in the liver. International Journal of Biochemistry and Cell Biology. 2008;40(8):1437–1442. - PubMed
-
- Feitelson MA. Hepatitis C Virus: From Laboratory to Clinic. Cambridge, UK: Cambridge University Press; 2004.
-
- Czepiel J, Biesiada G, Mach T. Viral hepatitis C. Polskie Archiwum Medycyny Wewnetrznej. 2008;118(12):734–740. - PubMed
-
- Oka K, Nagano-Fujii M, Yoshida I, et al. Hepatitis C virus core protein selectively inhibits synthesis and accumulation of p21/Waf1 and certain nuclear proteins. Microbiology and Immunology. 2003;47(6):429–438. - PubMed
-
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nature Reviews Microbiology. 2007;5(6):453–463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

