Th subset balance in lupus nephritis
- PMID: 21904445
- PMCID: PMC3163408
- DOI: 10.1155/2011/980286
Th subset balance in lupus nephritis
Abstract
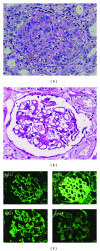
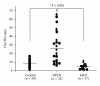
Lupus nephritis, which has various histological patterns and variable clinical outcomes, is one of the most important complications of systemic lupus nephritis (SLE). This pathogenetic mechanism in each histologically different type of lupus nephritis (LN) remains unclear. Although SLE is suggested to be a Th2-driven disease, elevation of both Th1 and Th2 cytokines occurs in both humans and mice, suggesting that SLE is a complex disease driven by different lymphocyte subsets with high heterogeneity of clinical manifestations and organ involvement. Recent findings in LN elucidate an essential role for the Th1, IL-17 producing T cells and Th17 cells in the development of diffuse proliferative lupus nephritis (DPLN), and Th2 cytokine in that of membranous lupus nephritis (MLN). These data support the hypothesis that individual Th1/Th2 balance is one of the critical determinants for histopathology of LN.
Figures


References
-
- Cochrane CG, Koffler D. Immune complex disease in experimental animals and man. Advances in Immunology. 1973;16(C):185–264. - PubMed
-
- Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85(3):303–306. - PubMed
-
- Churg J, Bernstein J, Glassock RJ. Lupus nephritis. In: Churg J, Bernstein J, Glassock RJ, editors. Renal Disease: Classification and Atlas of Glomerular Disease. New York, NY, USA: Igaku-Shoin Medical; 1995. p. 151.
-
- Dixon FJ. The pathogenesis of glomerulonephritis. The American Journal of Medicine. 1968;44(4):493–498. - PubMed
-
- Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney International. 2004;65(2):521–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

