Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review
- PMID: 21904528
- PMCID: PMC3161244
- DOI: 10.3389/fphar.2011.00049
Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review
Abstract
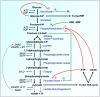
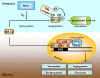
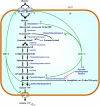
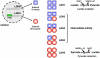
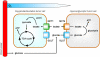
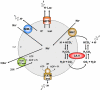
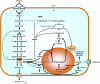
CANCER IS A METABOLIC DISEASE AND THE SOLUTION OF TWO METABOLIC EQUATIONS: to produce energy with limited resources and to fulfill the biosynthetic needs of proliferating cells. Both equations are solved when glycolysis is uncoupled from oxidative phosphorylation in the tricarboxylic acid cycle, a process known as the glycolytic switch. This review addresses in a comprehensive manner the main molecular events accounting for high-rate glycolysis in cancer. It starts from modulation of the Pasteur Effect allowing short-term adaptation to hypoxia, highlights the key role exerted by the hypoxia-inducible transcription factor HIF-1 in long-term adaptation to hypoxia, and summarizes the current knowledge concerning the necessary involvement of aerobic glycolysis (the Warburg effect) in cancer cell proliferation. Based on the many observations positioning glycolysis as a central player in malignancy, the most advanced anticancer treatments targeting tumor glycolysis are briefly reviewed.
Keywords: HIF-1; Warburg effect; biosynthesis; cancer; cataplerosis; glycolytic switch; hypoxia; metabolism.
Figures
References
-
- Abbate F., Casini A., Owa T., Scozzafava A., Supuran C. T. (2004). Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg. Med. Chem. Lett. 14, 217–223 10.1016/j.bmcl.2003.09.064 - DOI - PubMed
-
- Atsumi T., Chesney J., Metz C., Leng L., Donnelly S., Makita Z., Mitchell R., Bucala R. (2002). High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 62, 5881–5887 - PubMed
-
- Battke C., Kremmer E., Mysliwietz J., Gondi G., Dumitru C., Brandau S., Lang S., Vullo D., Supuran C., Zeidler R. (2011). Generation and characterization of the first inhibitory antibody targeting tumour-associated carbonic anhydrase XII. Cancer Immunol. Immunother. 60, 649–658 10.1007/s00262-011-0979-5 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

