Longitudinal analysis of arterial blood pressure and heart rate response to acute behavioral stress in rats with type 1 diabetes mellitus and in age-matched controls
- PMID: 21904530
- PMCID: PMC3163305
- DOI: 10.3389/fphys.2011.00053
Longitudinal analysis of arterial blood pressure and heart rate response to acute behavioral stress in rats with type 1 diabetes mellitus and in age-matched controls
Abstract
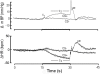
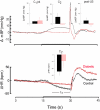
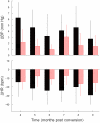
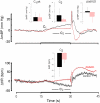
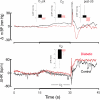
We recorded via telemetry the arterial blood pressure (BP) and heart rate (HR) response to classical conditioning following the spontaneous onset of autoimmune diabetes in BBDP/Wor rats vs. age-matched, diabetes-resistant control (BBDR/Wor) rats. Our purpose was to evaluate the autonomic regulatory responses to an acute stress in a diabetic state of up to 12 months duration. The stress was a 15-s pulsed tone (CS+) followed by a 0.5-s tail shock. The initial, transient increase in BP (i.e., the "first component," or C(1)), known to be derived from an orienting response and produced by a sympathetic increase in peripheral resistance, was similar in diabetic and control rats through ∼9 months of diabetes; it was smaller in diabetic rats 10 months after diabetes onset. Weakening of the C(1) BP increase in rats that were diabetic for >10 months is consistent with the effects of sympathetic neuropathy. A longer-latency, smaller, but sustained "second component" (C(2)) conditional increase in BP, that is acquired as a rat learns the association between CS+ and the shock, and which results from an increase in cardiac output, was smaller in the diabetic vs. control rats starting from the first month of diabetes. A concomitant HR slowing was also smaller in diabetic rats. The difference in the C(2) BP increase, as observed already during the first month of diabetes, is probably secondary to the effects of hyperglycemia upon myocardial metabolism and contractile function, but it may also result from effects on cognition. The small HR slowing concomitant with the C(2) pressor event is probably secondary to differences in baroreflex activation or function, though parasympathetic dysfunction may contribute later in the duration of diabetes. The nearly immediate deficit after disease onset in the C(2) response indicates that diabetes alters BP and HR responses to external challenges prior to the development of structural changes in the vasculature or autonomic nerves.
Keywords: Pavlovian (classical) conditioning; anxiety; autonomic nervous system; cardiovascular system; dysautonomia; telemetry.
Figures
Similar articles
-
A comparison of the autonomic nervous control of the heart during classical aversive vs appetitive conditioning in dog.J Auton Nerv Syst. 1985 Jun;13(2):125-36. doi: 10.1016/0165-1838(85)90029-3. J Auton Nerv Syst. 1985. PMID: 4020033
-
Circadian Variations in Blood Pressure, Heart Rate, and HR-BP Cross-Correlation Coefficient during Progression of Diabetes Mellitus in Rat.Int J Hypertens. 2011;2011:738689. doi: 10.4061/2011/738689. Epub 2011 Apr 19. Int J Hypertens. 2011. PMID: 21629872 Free PMC article.
-
Differential acquisition of specific components of a classically conditioned arterial blood pressure response in rat.Am J Physiol Regul Integr Comp Physiol. 2005 Sep;289(3):R784-8. doi: 10.1152/ajpregu.00018.2005. Epub 2005 Apr 28. Am J Physiol Regul Integr Comp Physiol. 2005. PMID: 15860652
-
Squatting, a posture test for studying cardiovascular autonomic neuropathy in diabetes.Diabetes Metab. 2011 Dec;37(6):489-96. doi: 10.1016/j.diabet.2011.09.004. Epub 2011 Nov 8. Diabetes Metab. 2011. PMID: 22071282 Review.
-
Effect of aging on baroreflex function in humans.Am J Physiol Regul Integr Comp Physiol. 2007 Jul;293(1):R3-R12. doi: 10.1152/ajpregu.00031.2007. Epub 2007 Apr 18. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 17442786 Review.
Cited by
-
Maternal separation diminishes α-adrenergic receptor density and function in renal vasculature from male Wistar-Kyoto rats.Am J Physiol Renal Physiol. 2017 Jul 1;313(1):F47-F54. doi: 10.1152/ajprenal.00591.2016. Epub 2017 Mar 22. Am J Physiol Renal Physiol. 2017. PMID: 28331064 Free PMC article.
-
Vascular response of ruthenium tetraamines in aortic ring from normotensive rats.Arq Bras Cardiol. 2015 Mar;104(3):185-94. doi: 10.5935/abc.20140189. Epub 2014 Dec 9. Arq Bras Cardiol. 2015. PMID: 25494016 Free PMC article.
-
Extended longitudinal analysis of arterial pressure and heart rate control in unanesthetized rats with type 1 diabetes.Auton Neurosci. 2012 Sep 25;170(1-2):20-9. doi: 10.1016/j.autneu.2012.06.006. Epub 2012 Jul 17. Auton Neurosci. 2012. PMID: 22809731 Free PMC article.
References
-
- Addicks K., Boy C., Rosen P. (1993). Sympathetic autonomic neuropathy in the heart of the spontaneous diabetic BB rat. Ann. Anat. 175, 253–257 - PubMed
-
- Brown D. R., Li S.-G., Lawler J. E., Randall D. C. (1999). Sympathetic control of BP and BP variability in borderline hypertensive rats on high- vs. low-salt diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 277, R650–R657 - PubMed
-
- Burgess D., Randall D. C., Stocker S. D. (2009). Influence of diabetes on baroreflex (BR) sensitivity of sympathetic nerve activity (SNA). FASEB J. 23, 610.6.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

