Structural and Functional Consequences Induced by Post-Translational Modifications in α-Defensins
- PMID: 21904558
- PMCID: PMC3163396
- DOI: 10.1155/2011/594723
Structural and Functional Consequences Induced by Post-Translational Modifications in α-Defensins
Abstract
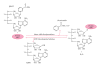
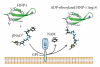
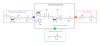
HNP-1 is an antimicrobial peptide that undergoes proteolytic cleavage to become a mature peptide. This process represents the mechanism commonly used by the cells to obtain a fully active antimicrobial peptide. In addition, it has been recently described that HNP-1 is recognized as substrate by the arginine-specific ADP-ribosyltransferase-1. Arginine-specific mono-ADP-ribosylation is an enzyme-catalyzed post-translational modification in which NAD(+) serves as donor of the ADP-ribose moiety, which is transferred to the guanidino group of arginines in target proteins. While the arginine carries one positive charge, the ADP-ribose is negatively charged at the phosphate moieties at physiological pH. Therefore, the attachment of one or more ADP-ribose units results in a marked change of cationicity. ADP-ribosylation of HNP-1 drastically reduces its cytotoxic and antibacterial activities. While the chemotactic activity of HNP-1 remains unaltered, its ability to induce interleukin-8 production is enhanced. The arginine 14 of HNP-1 modified by the ADP-ribose is in some cases processed into ornithine, perhaps representing a different modality in the regulation of HNP-1 activities.
Figures
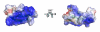

References
-
- Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annual Review of Immunology. 1993;11:105–128. - PubMed
-
- Oppenheim FG, Xu T, McMillian FM, et al. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. Journal of Biological Chemistry. 1988;263(16):7472–7477. - PubMed
-
- Schittek B, Hipfel R, Sauer B, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nature Immunology. 2001;2(12):1133–1137. - PubMed
-
- Peña SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. Journal of Immunology. 1997;158(6):2680–2688. - PubMed
-
- Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel β-defensin from human plasma. FEBS Letters. 1995;368(2):331–335. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

