Intradermal indocyanine green for in vivo fluorescence laser scanning microscopy of human skin: a pilot study
- PMID: 21904601
- PMCID: PMC3164142
- DOI: 10.1371/journal.pone.0023972
Intradermal indocyanine green for in vivo fluorescence laser scanning microscopy of human skin: a pilot study
Abstract
Background: In clinical diagnostics, as well as in routine dermatology, the increased need for non-invasive diagnosis is currently satisfied by reflectance laser scanning microscopy. However, this technique has some limitations as it relies solely on differences in the reflection properties of epidermal and dermal structures. To date, the superior method of fluorescence laser scanning microscopy is not generally applied in dermatology and predominantly restricted to fluorescein as fluorescent tracer, which has a number of limitations. Therefore, we searched for an alternative fluorophore matching a novel skin imaging device to advance this promising diagnostic approach.
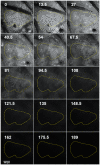
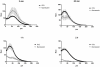
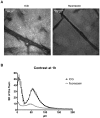
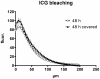
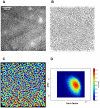
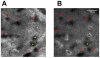
Methodology/principal findings: Using a Vivascope®-1500 Multilaser microscope, we found that the fluorophore Indocyanine-Green (ICG) is well suited as a fluorescent marker for skin imaging in vivo after intradermal injection. ICG is one of few fluorescent dyes approved for use in humans. Its fluorescence properties are compatible with the application of a near-infrared laser, which penetrates deeper into the tissue than the standard 488 nm laser for fluorescein. ICG-fluorescence turned out to be much more stable than fluorescein in vivo, persisting for more than 48 hours without significant photobleaching whereas fluorescein fades within 2 hours. The well-defined intercellular staining pattern of ICG allows automated cell-recognition algorithms, which we accomplished with the free software CellProfiler, providing the possibility of quantitative high-content imaging. Furthermore, we demonstrate the superiority of ICG-based fluorescence microscopy for selected skin pathologies, including dermal nevi, irritant contact dermatitis and necrotic skin.
Conclusions/significance: Our results introduce a novel in vivo skin imaging technique using ICG, which delivers a stable intercellular fluorescence signal ideal for morphological assessment down to sub-cellular detail. The application of ICG in combination with the near infrared laser opens new ways for minimal-invasive diagnosis and monitoring of skin disorders.
Conflict of interest statement
Figures
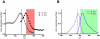




References
-
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology. Lasers Med Sci. 2007;22:73–82. - PubMed
-
- Ulrich M, Stockfleth E, Roewert-Huber J, Astner S. Noninvasive diagnostic tools for nonmelanoma skin cancer. Br J Dermatol. 2007;157(Suppl 2):56–58. - PubMed
-
- Gonzalez S, Gilaberte-Calzada Y. In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. Int J Cosmet Sci. 2008;30:1–17. - PubMed
-
- Ulrich M, Krueger-Corcoran D, Roewert-Huber J, Sterry W, Stockfleth E, et al. Reflectance confocal microscopy for noninvasive monitoring of therapy and detection of subclinical actinic keratoses. Dermatology. 2010;220:15–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

