Protein kinases and phosphatases in the control of cell fate
- PMID: 21904669
- PMCID: PMC3166778
- DOI: 10.4061/2011/329098
Protein kinases and phosphatases in the control of cell fate
Abstract
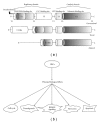
Protein phosphorylation controls many aspects of cell fate and is often deregulated in pathological conditions. Several recent findings have provided an intriguing insight into the spatial regulation of protein phosphorylation across different subcellular compartments and how this can be finely orchestrated by specific kinases and phosphatases. In this review, the focus will be placed on (i) the phosphoinositide 3-kinase (PI3K) pathway, specifically on the kinases Akt and mTOR and on the phosphatases PP2a and PTEN, and on (ii) the PKC family of serine/threonine kinases. We will look at general aspects of cell physiology controlled by these kinases and phosphatases, highlighting the signalling pathways that drive cell division, proliferation, and apoptosis.
Figures

References
-
- Julien SG, Dubé N, Hardy S, Tremblay ML. Inside the human cancer tyrosine phosphatome. Nature Reviews Cancer. 2011;11(1):35–49. - PubMed
-
- Ruela-de-Sousa RR, Queiroz KC, Peppelenbosch MP, Fuhler GM. Reversible phosphorylation in haematological malignancies: potential role for protein tyrosine phosphatases in treatment? Biochimica et Biophysica Acta. 2010;1806(2):287–303. - PubMed
-
- Zhang H, Zha X, Tan Y, et al. Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. Journal of Biological Chemistry. 2002;277(42):39379–39387. - PubMed
-
- Bauman AL, Scott JD. Kinase- and phosphatase-anchoring proteins: Harnessing the dynamic duo. Nature Cell Biology. 2002;4(8):E203–E206. - PubMed
-
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

