Stem cell technology for neurodegenerative diseases
- PMID: 21905078
- PMCID: PMC3177143
- DOI: 10.1002/ana.22487
Stem cell technology for neurodegenerative diseases
Abstract
Over the past 20 years, stem cell technologies have become an increasingly attractive option to investigate and treat neurodegenerative diseases. In the current review, we discuss the process of extending basic stem cell research into translational therapies for patients suffering from neurodegenerative diseases. We begin with a discussion of the burden of these diseases on society, emphasizing the need for increased attention toward advancing stem cell therapies. We then explain the various types of stem cells utilized in neurodegenerative disease research, and outline important issues to consider in the transition of stem cell therapy from bench to bedside. Finally, we detail the current progress regarding the applications of stem cell therapies to specific neurodegenerative diseases, focusing on Parkinson disease, Huntington disease, Alzheimer disease, amyotrophic lateral sclerosis, and spinal muscular atrophy. With a greater understanding of the capacity of stem cell technologies, there is growing public hope that stem cell therapies will continue to progress into realistic and efficacious treatments for neurodegenerative diseases.
Copyright © 2011 American Neurological Association.
Figures
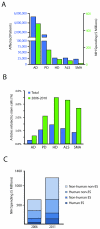


Comment in
-
Stem cell therapy for Parkinson's disease.Ann Neurol. 2012 Feb;71(2):283; author reply 284. doi: 10.1002/ana.22676. Ann Neurol. 2012. PMID: 22368000 No abstract available.
References
-
- Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. - PubMed
-
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. - PubMed
-
- Gaspard N, Vanderhaeghen P. Mechanisms of neural specification from embryonic stem cells. Curr Opin Neurobiol. 2010;20:37–43. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

