Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy
- PMID: 21905079
- PMCID: PMC3177145
- DOI: 10.1002/ana.22479
Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy
Abstract
Objective: Enduring, abnormal expression and function of the ion channel hyperpolarization-activated cyclic adenosine monophosphate gated channel type 1 (HCN1) occurs in temporal lobe epilepsy (TLE). We examined the underlying mechanisms, and investigated whether interfering with these mechanisms could modify disease course.
Methods: Experimental TLE was provoked by kainic acid-induced status epilepticus (SE). HCN1 channel repression was examined at mRNA, protein, and functional levels. Chromatin immunoprecipitation was employed to identify the transcriptional mechanism of repressed HCN1 expression, and the basis for their endurance. Physical interaction of the repressor, NRSF, was abolished using decoy oligodeoxynucleotides (ODNs). Video/electroencephalographic recordings were performed to assess the onset and initial pattern of spontaneous seizures.
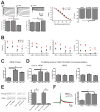
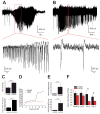
Results: Levels of NRSF and its physical binding to the Hcn1 gene were augmented after SE, resulting in repression of HCN1 expression and HCN1-mediated currents (I(h) ), and reduced I(h) -dependent resonance in hippocampal CA1 pyramidal cell dendrites. Chromatin changes typical of enduring, epigenetic gene repression were apparent at the Hcn1 gene within a week after SE. Administration of decoy ODNs comprising the NRSF DNA-binding sequence (neuron restrictive silencer element [NRSE]), in vitro and in vivo, reduced NRSF binding to Hcn1, prevented its repression, and restored I(h) function. In vivo, decoy NRSE ODN treatment restored theta rhythm and altered the initial pattern of spontaneous seizures.
Interpretation: Acquired HCN1 channelopathy derives from NRSF-mediated transcriptional repression that endures via chromatin modification and may provide insight into the mechanisms of a number of channelopathies that coexist with, and may contribute to, the conversion of a normal brain into an epileptic one.
Copyright © 2011 American Neurological Association.
Figures




References
-
- Pitkanen A, Kharatishvili I, Karhunen H, et al. Epileptogenesis in experimental models. Epilepsia. 2007;48(Suppl 2):13–20. - PubMed
-
- Lukasiuk K, Kontula L, Pitkanen A. cDNA profiling of epileptogenesis in the rat brain. Eur J Neurosci. 2003;17:271–279. - PubMed
-
- Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. - PubMed
-
- Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

