Erythropoietin (EPO) in acute kidney injury
- PMID: 21906325
- PMCID: PMC3159901
- DOI: 10.1186/2110-5820-1-3
Erythropoietin (EPO) in acute kidney injury
Abstract
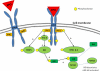
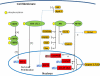
Erythropoietin (EPO) is a 30.4 kDa glycoprotein produced by the kidney, and is mostly well-known for its physiological function in regulating red blood cell production in the bone marrow. Accumulating evidence, however, suggests that EPO has additional organ protective effects, which may be useful in the prevention or treatment of acute kidney injury. These protective mechanisms are multifactorial in nature and include inhibition of apoptotic cell death, stimulation of cellular regeneration, inhibition of deleterious pathways, and promotion of recovery.In this article, we review the physiology of EPO, assess previous work that supports the role of EPO as a general tissue protective agent, and explain the mechanisms by which it may achieve this tissue protective effect. We then focus on experimental and clinical data that suggest that EPO has a kidney protective effect.
Figures
References
-
- Boissel JP, Lee WR, Presnell SR, Cohen FE, Bunn HF. Erythropoietin structure-function relationships. Mutant proteins that test a model of tertiary structure. J Biol Chem. 1993;268:15983–93. - PubMed
-
- Catlin DH, Breidbach A, Elliott S, Glaspy J. Comparison of the isoelectric focusing patterns of darbepoetin alfa, recombinant human erythropoietin, and endogenous erythropoietin from human urine. Clin Chem. 2002;48:2057–9. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

