Characterization of diverse natural variants of CYP102A1 found within a species of Bacillus megaterium
- PMID: 21906327
- PMCID: PMC3159907
- DOI: 10.1186/2191-0855-1-1
Characterization of diverse natural variants of CYP102A1 found within a species of Bacillus megaterium
Abstract
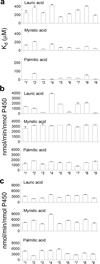
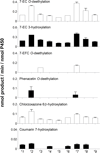
An extreme diversity of substrates and catalytic reactions of cytochrome P450 (P450) enzymes is considered to be the consequence of evolutionary adaptation driven by different metabolic or environmental demands. Here we report the presence of numerous natural variants of P450 BM3 (CYP102A1) within a species of Bacillus megaterium. Extensive amino acid substitutions (up to 5% of the total 1049 amino acid residues) were identified from the variants. Phylogenetic analyses suggest that this P450 gene evolve more rapidly than the rRNA gene locus. It was found that key catalytic residues in the substrate channel and active site are retained. Although there were no apparent variations in hydroxylation activity towards myristic acid (C14) and palmitic acid (C16), the hydroxylation rates of lauric acid (C12) by the variants varied in the range of >25-fold. Interestingly, catalytic activities of the variants are promiscuous towards non-natural substrates including human P450 substrates. It can be suggested that CYP102A1 variants can acquire new catalytic activities through site-specific mutations distal to the active site.
Figures


Similar articles
-
Choose Your Own Adventure: A Comprehensive Database of Reactions Catalyzed by Cytochrome P450 BM3 Variants.ACS Catal. 2024 Mar 29;14(8):5560-5592. doi: 10.1021/acscatal.4c00086. eCollection 2024 Apr 19. ACS Catal. 2024. PMID: 38660610 Free PMC article. Review.
-
Expression, purification, and characterization of Bacillus subtilis cytochromes P450 CYP102A2 and CYP102A3: flavocytochrome homologues of P450 BM3 from Bacillus megaterium.Biochemistry. 2004 May 11;43(18):5474-87. doi: 10.1021/bi035904m. Biochemistry. 2004. PMID: 15122913
-
Engineering cytochrome P450 BM3 of Bacillus megaterium for terminal oxidation of palmitic acid.J Biotechnol. 2014 Aug 20;184:17-26. doi: 10.1016/j.jbiotec.2014.05.002. Epub 2014 May 14. J Biotechnol. 2014. PMID: 24833423
-
Selective hydroxylation of highly branched fatty acids and their derivatives by CYP102A1 from Bacillus megaterium.Chembiochem. 2006 May;7(5):789-94. doi: 10.1002/cbic.200500444. Chembiochem. 2006. PMID: 16566047
-
P450(BM3) (CYP102A1): connecting the dots.Chem Soc Rev. 2012 Feb 7;41(3):1218-60. doi: 10.1039/c1cs15192d. Epub 2011 Oct 18. Chem Soc Rev. 2012. PMID: 22008827 Review.
Cited by
-
Enzymatic Production of 3-OH Phlorizin, a Possible Bioactive Polyphenol from Apples, by Bacillus megaterium CYP102A1 via Regioselective Hydroxylation.Antioxidants (Basel). 2021 Aug 23;10(8):1327. doi: 10.3390/antiox10081327. Antioxidants (Basel). 2021. PMID: 34439575 Free PMC article.
-
Oleic acid based experimental evolution of Bacillus megaterium yielding an enhanced P450 BM3 variant.BMC Biotechnol. 2022 Jul 13;22(1):20. doi: 10.1186/s12896-022-00750-w. BMC Biotechnol. 2022. PMID: 35831844 Free PMC article.
-
Choose Your Own Adventure: A Comprehensive Database of Reactions Catalyzed by Cytochrome P450 BM3 Variants.ACS Catal. 2024 Mar 29;14(8):5560-5592. doi: 10.1021/acscatal.4c00086. eCollection 2024 Apr 19. ACS Catal. 2024. PMID: 38660610 Free PMC article. Review.
-
A Novel Statin Compound from Monacolin J Produced Using CYP102A1-Catalyzed Regioselective C-Hydroxylation.Pharmaceuticals (Basel). 2021 Sep 26;14(10):981. doi: 10.3390/ph14100981. Pharmaceuticals (Basel). 2021. PMID: 34681205 Free PMC article.
-
The Versatile Biocatalyst of Cytochrome P450 CYP102A1: Structure, Function, and Engineering.Molecules. 2023 Jul 12;28(14):5353. doi: 10.3390/molecules28145353. Molecules. 2023. PMID: 37513226 Free PMC article. Review.
References
-
- Aharoni A, Gaidukov L, Khersonsky O, McQ Gould S, Roodveldt C, Tawfik DS. The 'evolvability' of promiscuous protein functions. Nat Genet. 2005;37:73–76. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Boddupalli SS, Estabrook RW, Peterson JA. Fatty acid monooxygenation by cytochrome P-450BM-3. J Biol Chem. 1990;265:4233–4239. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

