Effects of SDF-1-CXCR4 signaling on microRNA expression and tumorigenesis in estrogen receptor-alpha (ER-α)-positive breast cancer cells
- PMID: 21906588
- PMCID: PMC3334320
- DOI: 10.1016/j.yexcr.2011.08.016
Effects of SDF-1-CXCR4 signaling on microRNA expression and tumorigenesis in estrogen receptor-alpha (ER-α)-positive breast cancer cells
Abstract
The majority of breast cancer cases ultimately become unresponsive to endocrine therapies, and this progression of breast cancer from hormone-responsive to hormone-independent represents an area in need of further research. Additionally, hormone-independent carcinomas are characterized as being more aggressive and metastatic, key features of more advanced disease. Having previously shown the ability of the stromal-cell derived factor-1 (SDF-1)-CXCR4 signaling axis to promote primary tumorigenesis and hormone independence by overexpressing CXCR4 in MCF-7 cells, in this study we further examined the role of SDF-1/CXCR4 in the endogenously CXCR4-positive, estrogen receptor α (ER-α)-positive breast carcinoma cell line, MDA-MB-361. In addition to regulating estrogen-induced and hormone-independent tumor growth, CXCR4 signaling stimulated the epithelial-to-mesenchymal transition, evidenced by decreased CDH1 expression following SDF-1 treatment. Furthermore, inhibition of CXCR4 with the small molecule inhibitor AMD3100 induced CDH1 gene expression and inhibited CDH2 gene expression in MDA-MB-361 cells. Further, exogenous SDF-1 treatment induced ER-α-phosphorylation in both MDA-MB-361 and MCF-7-CXCR4 cells, demonstrating ligand-independent activation of ER-α through CXCR4 crosstalk. qPCR microRNA array analyses of the MDA-MB-361 and MCF-7-CXCR4 cell lines revealed changes in microRNA expression profiles induced by SDF-1, consistent with a more advanced disease phenotype and further supporting our hypothesis that the SDF-1/CXCR4 signaling axis drives ER-α-positive breast cancer cells to a hormone independent and more aggressive phenotype. In this first demonstration of SDF-1-CXCR4-induced microRNAs in breast cancer, we suggest that this signaling axis may promote tumorigenesis via microRNA regulation. These findings represent future potential therapeutic targets for the treatment of hormone-independent and endocrine-resistant breast cancer.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
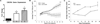
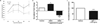

Similar articles
-
Effects of human mesenchymal stem cells on ER-positive human breast carcinoma cells mediated through ER-SDF-1/CXCR4 crosstalk.Mol Cancer. 2010 Nov 18;9:295. doi: 10.1186/1476-4598-9-295. Mol Cancer. 2010. PMID: 21087507 Free PMC article.
-
Overlapping and distinct role of CXCR7-SDF-1/ITAC and CXCR4-SDF-1 axes in regulating metastatic behavior of human rhabdomyosarcomas.Int J Cancer. 2010 Dec 1;127(11):2554-68. doi: 10.1002/ijc.25245. Int J Cancer. 2010. PMID: 20162608 Free PMC article.
-
Positive feedback activation of estrogen receptors by the CXCL12-CXCR4 pathway.Cancer Res. 2009 Jul 15;69(14):5793-800. doi: 10.1158/0008-5472.CAN-08-4924. Epub 2009 Jul 7. Cancer Res. 2009. PMID: 19584281
-
The CXCR4/SDF-1 chemokine axis: a potential therapeutic target for bone metastases?Curr Pharm Des. 2010;16(11):1284-90. doi: 10.2174/138161210791034012. Curr Pharm Des. 2010. PMID: 20166978 Review.
-
CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies?Clin Cancer Res. 2011 Apr 15;17(8):2074-80. doi: 10.1158/1078-0432.CCR-10-2636. Epub 2011 Feb 24. Clin Cancer Res. 2011. PMID: 21349998 Free PMC article. Review.
Cited by
-
High-level expression of CXCR4 in breast cancer is associated with early distant and bone metastases.Tumour Biol. 2014 Feb;35(2):1581-8. doi: 10.1007/s13277-013-1218-9. Epub 2013 Oct 8. Tumour Biol. 2014. PMID: 24101191
-
Microenvironment and endocrine resistance in breast cancer: Friend or foe?World J Clin Oncol. 2015 Dec 10;6(6):207-11. doi: 10.5306/wjco.v6.i6.207. World J Clin Oncol. 2015. PMID: 26677432 Free PMC article.
-
CXCR4/CXCL12 Signaling and Protumor Macrophages in Primary Tumors and Sentinel Lymph Nodes Are Involved in Luminal B Breast Cancer Progression.Dis Markers. 2018 Apr 16;2018:5018671. doi: 10.1155/2018/5018671. eCollection 2018. Dis Markers. 2018. PMID: 29849822 Free PMC article.
-
Inhibiting metastatic breast cancer cell migration via the synergy of targeted, pH-triggered siRNA delivery and chemokine axis blockade.Mol Pharm. 2014 Mar 3;11(3):755-65. doi: 10.1021/mp4004699. Epub 2014 Feb 12. Mol Pharm. 2014. PMID: 24467226 Free PMC article.
-
SDF-1α Facilitates Mesenchymal Stem Cells to Induce Regulatory B Cell Differentiation from Patients with Immune Thrombocytopenia.Stem Cells Int. 2021 Nov 8;2021:3254488. doi: 10.1155/2021/3254488. eCollection 2021. Stem Cells Int. 2021. PMID: 34790240 Free PMC article.
References
-
- Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Seminars in cancer biology. 2004;14:171–179. - PubMed
-
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. - PubMed
-
- Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer research. 2004;64:8604–8612. - PubMed
-
- Jordan VC, Gottardis MM, Robinson SP, Friedl A. Immune-deficient animals to study "hormone-dependent" breast and endometrial cancer. Journal of steroid biochemistry. 1989;34:169–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

