Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions
- PMID: 21906648
- PMCID: PMC3224191
- DOI: 10.1016/j.vaccine.2011.08.089
Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions
Abstract
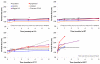
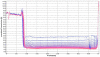
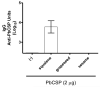
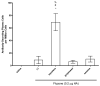
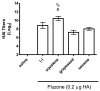
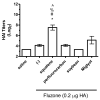
Squalene-based oil-in-water emulsions have been used for years in some seasonal and pandemic influenza vaccines. However, concerns have been expressed regarding squalene source and potential biological activities. Little information is available regarding the immunomodulatory activity of squalene in comparison with other metabolizable oils in the context of oil-in-water emulsions formulated with vaccines. The present work describes the manufacture and physical characterization of emulsions composed of different classes of oils, including squalene, long chain triglycerides, a medium chain triglyceride, and a perfluorocarbon, all emulsified with egg phosphatidylcholine. Some differences were apparent among the non-squalene oils in terms of emulsion stability, including higher size polydispersity in the perfluorocarbon emulsion, more rapid visual instability at 60°C for the long-chain triglyceride and perfluorocarbon emulsions, and an increased creaming rate in the medium-chain triglyceride emulsion at 60°C as detected by laser scattering optical profiling. The biological activity of each of these emulsions was compared when formulated with either a recombinant malaria antigen or a split-virus inactivated influenza vaccine. Overall, vaccines containing the squalene emulsion elicited higher antibody titers and more abundant long-lived plasma cells than vaccines containing emulsions based on other oils. Since squalene-based emulsions show higher adjuvant potency compared to the other oils tested, non-squalene oils may be more suitable as carriers of amphiphilic or hydrophobic immunostimulatory molecules (such as TLR agonists) rather than as stand-alone adjuvants.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

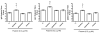





Similar articles
-
Immunomodulatory and physical effects of phospholipid composition in vaccine adjuvant emulsions.AAPS PharmSciTech. 2012 Jun;13(2):498-506. doi: 10.1208/s12249-012-9771-x. Epub 2012 Mar 14. AAPS PharmSciTech. 2012. PMID: 22415641 Free PMC article.
-
Adjuvanted pandemic influenza vaccine: variation of emulsion components affects stability, antigen structure, and vaccine efficacy.Influenza Other Respir Viruses. 2013 Sep;7(5):815-26. doi: 10.1111/irv.12031. Epub 2012 Nov 5. Influenza Other Respir Viruses. 2013. PMID: 23122325 Free PMC article.
-
A novel oil-in-water emulsion as a potential adjuvant for influenza vaccine: development, characterization, stability and in vivo evaluation.Int J Pharm. 2014 Jul 1;468(1-2):187-95. doi: 10.1016/j.ijpharm.2014.04.003. Epub 2014 Apr 4. Int J Pharm. 2014. PMID: 24704309
-
Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion.Expert Rev Vaccines. 2012 Mar;11(3):349-66. doi: 10.1586/erv.11.192. Expert Rev Vaccines. 2012. PMID: 22380826 Review.
-
An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants.Expert Rev Vaccines. 2013 Jul;12(7):747-58. doi: 10.1586/14760584.2013.811188. Expert Rev Vaccines. 2013. PMID: 23885820 Review.
Cited by
-
Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes.Clin Exp Vaccine Res. 2015 Jan;4(1):23-45. doi: 10.7774/cevr.2015.4.1.23. Epub 2015 Jan 30. Clin Exp Vaccine Res. 2015. PMID: 25648619 Free PMC article. Review.
-
Research progress on emulsion vaccine adjuvants.Heliyon. 2024 Jan 12;10(3):e24662. doi: 10.1016/j.heliyon.2024.e24662. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38317888 Free PMC article. Review.
-
Melanoma vaccines and modulation of the immune system in the clinical setting: building from new realities.Front Immunol. 2012 May 4;3:103. doi: 10.3389/fimmu.2012.00103. eCollection 2012. Front Immunol. 2012. PMID: 22566975 Free PMC article.
-
Semi-synthetic terpenoids with differential adjuvant properties as sustainable replacements for shark squalene in vaccine emulsions.NPJ Vaccines. 2023 Feb 16;8(1):14. doi: 10.1038/s41541-023-00608-y. NPJ Vaccines. 2023. PMID: 36797262 Free PMC article.
-
Immunogenicity of Different Types of Adjuvants and Nano-Adjuvants in Veterinary Vaccines: A Comprehensive Review.Vaccines (Basel). 2023 Feb 16;11(2):453. doi: 10.3390/vaccines11020453. Vaccines (Basel). 2023. PMID: 36851331 Free PMC article. Review.
References
-
- Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21:201–30. - PubMed
-
- Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–22. - PubMed
-
- Asa PB, Cao Y, Garry RF. Antibodies to squalene in Gulf War Syndrome. Exp Mol Pathol. 2000;68:55–64. - PubMed
-
- Asa PB, Wilson RB, Garry RF. Antibodies to squalene in recipients of anthrax vaccine. Exp Mol Pathol. 2002;73:19–27. - PubMed
-
- Alving CR, Grabenstein JD. Letter to the Editor. Exp Mol Pathol. 2000;68:196–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

