Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions
- PMID: 21906648
- PMCID: PMC3224191
- DOI: 10.1016/j.vaccine.2011.08.089
Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions
Abstract
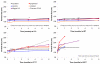
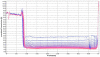
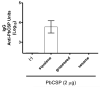
Squalene-based oil-in-water emulsions have been used for years in some seasonal and pandemic influenza vaccines. However, concerns have been expressed regarding squalene source and potential biological activities. Little information is available regarding the immunomodulatory activity of squalene in comparison with other metabolizable oils in the context of oil-in-water emulsions formulated with vaccines. The present work describes the manufacture and physical characterization of emulsions composed of different classes of oils, including squalene, long chain triglycerides, a medium chain triglyceride, and a perfluorocarbon, all emulsified with egg phosphatidylcholine. Some differences were apparent among the non-squalene oils in terms of emulsion stability, including higher size polydispersity in the perfluorocarbon emulsion, more rapid visual instability at 60°C for the long-chain triglyceride and perfluorocarbon emulsions, and an increased creaming rate in the medium-chain triglyceride emulsion at 60°C as detected by laser scattering optical profiling. The biological activity of each of these emulsions was compared when formulated with either a recombinant malaria antigen or a split-virus inactivated influenza vaccine. Overall, vaccines containing the squalene emulsion elicited higher antibody titers and more abundant long-lived plasma cells than vaccines containing emulsions based on other oils. Since squalene-based emulsions show higher adjuvant potency compared to the other oils tested, non-squalene oils may be more suitable as carriers of amphiphilic or hydrophobic immunostimulatory molecules (such as TLR agonists) rather than as stand-alone adjuvants.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

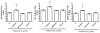
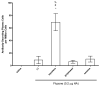

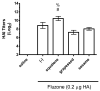

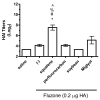



References
-
- Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21:201–30. - PubMed
-
- Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–22. - PubMed
-
- Asa PB, Cao Y, Garry RF. Antibodies to squalene in Gulf War Syndrome. Exp Mol Pathol. 2000;68:55–64. - PubMed
-
- Asa PB, Wilson RB, Garry RF. Antibodies to squalene in recipients of anthrax vaccine. Exp Mol Pathol. 2002;73:19–27. - PubMed
-
- Alving CR, Grabenstein JD. Letter to the Editor. Exp Mol Pathol. 2000;68:196–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

