Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function
- PMID: 21906802
- PMCID: PMC3190071
- DOI: 10.1016/j.biomaterials.2011.08.050
Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function
Abstract
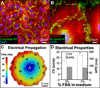
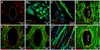
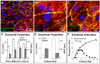
Recent advances in pluripotent stem cell research have provided investigators with potent sources of cardiogenic cells. However, tissue engineering methodologies to assemble cardiac progenitors into aligned, 3-dimensional (3D) myocardial tissues capable of physiologically relevant electrical conduction and force generation are lacking. In this study, we introduced 3D cell alignment cues in a fibrin-based hydrogel matrix to engineer highly functional cardiac tissues from genetically purified mouse embryonic stem cell-derived cardiomyocytes (CMs) and cardiovascular progenitors (CVPs). Procedures for CM and CVP derivation, purification, and functional differentiation in monolayer cultures were first optimized to yield robust intercellular coupling and maximize velocity of action potential propagation. A versatile soft-lithography technique was then applied to reproducibly fabricate engineered cardiac tissues with controllable size and 3D architecture. While purified CMs assembled into a functional 3D syncytium only when supplemented with supporting non-myocytes, purified CVPs differentiated into cardiomyocytes, smooth muscle, and endothelial cells, and autonomously supported the formation of functional cardiac tissues. After a total culture time similar to period of mouse embryonic development (21 days), the engineered cardiac tissues exhibited unprecedented levels of 3D organization and functional differentiation characteristic of native neonatal myocardium, including: 1) dense, uniformly aligned, highly differentiated and electromechanically coupled cardiomyocytes, 2) rapid action potential conduction with velocities between 22 and 25 cm/s, and 3) significant contractile forces of up to 2 mN. These results represent an important advancement in stem cell-based cardiac tissue engineering and provide the foundation for exploiting the exciting progress in pluripotent stem cell research in the future tissue engineering therapies for heart disease.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
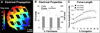

References
-
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. - PubMed
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. - PubMed
-
- Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science (New York, NY. 2008;322:1494–1497. - PubMed
-
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

