Hedgehog signaling regulates nociceptive sensitization
- PMID: 21906949
- PMCID: PMC3262399
- DOI: 10.1016/j.cub.2011.08.020
Hedgehog signaling regulates nociceptive sensitization
Abstract
Background: Nociceptive sensitization is a tissue damage response whereby sensory neurons near damaged tissue enhance their responsiveness to external stimuli. This sensitization manifests as allodynia (aversive withdrawal to previously nonnoxious stimuli) and/or hyperalgesia (exaggerated responsiveness to noxious stimuli). Although some factors mediating nociceptive sensitization are known, inadequacies of current analgesic drugs have prompted a search for additional targets.
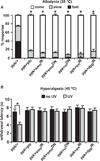
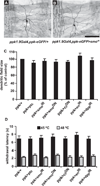
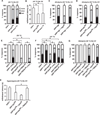
Results: Here we use a Drosophila model of thermal nociceptive sensitization to show that Hedgehog (Hh) signaling is required for both thermal allodynia and hyperalgesia following ultraviolet irradiation (UV)-induced tissue damage. Sensitization does not appear to result from developmental changes in the differentiation or arborization of nociceptive sensory neurons. Genetic analysis shows that Hh signaling acts in parallel to tumor necrosis factor (TNF) signaling to mediate allodynia and that distinct transient receptor potential (TRP) channels mediate allodynia and hyperalgesia downstream of these pathways. We also demonstrate a role for Hh in analgesic signaling in mammals. Intrathecal or peripheral administration of cyclopamine (CP), a specific inhibitor of Sonic Hedgehog signaling, blocked the development of analgesic tolerance to morphine (MS) or morphine antinociception in standard assays of inflammatory pain in rats and synergistically augmented and sustained morphine analgesia in assays of neuropathic pain.
Conclusions: We demonstrate a novel physiological role for Hh signaling, which has not previously been implicated in nociception. Our results also identify new potential therapeutic targets for pain treatment.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
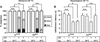

Comment in
-
Pain: A prickly solution?Nat Rev Neurosci. 2011 Oct 5;12(11):618. doi: 10.1038/nrn3124. Nat Rev Neurosci. 2011. PMID: 21971069 No abstract available.
References
-
- Woolf CJ. Overcoming obstacles to developing new analgesics. Nat. Med. 2010;16:1241–1247. - PubMed
-
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

