DJ-1 knock-down impairs astrocyte mitochondrial function
- PMID: 21907265
- PMCID: PMC3490195
- DOI: 10.1016/j.neuroscience.2011.08.016
DJ-1 knock-down impairs astrocyte mitochondrial function
Abstract
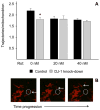
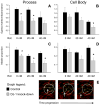
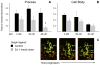
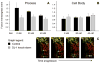
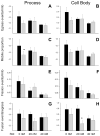
Mitochondrial dysfunction has long been implicated in the pathogenesis of Parkinson's disease (PD). PD brain tissues show evidence for mitochondrial respiratory chain Complex I deficiency. Pharmacological inhibitors of Complex I, such as rotenone, cause experimental parkinsonism. The cytoprotective protein DJ-1, whose deletion is sufficient to cause genetic PD, is also known to have mitochondria-stabilizing properties. We have previously shown that DJ-1 is over-expressed in PD astrocytes, and that DJ-1 deficiency impairs the capacity of astrocytes to protect co-cultured neurons against rotenone. Since DJ-1 modulated, astrocyte-mediated neuroprotection against rotenone may depend upon proper astrocytic mitochondrial functioning, we hypothesized that DJ-1 deficiency would impair astrocyte mitochondrial motility, fission/fusion dynamics, membrane potential maintenance, and respiration, both at baseline and as an enhancement of rotenone-induced mitochondrial dysfunction. In astrocyte-enriched cultures, we observed that DJ-1 knock-down reduced mitochondrial motility primarily in the cellular processes of both untreated and rotenone treated cells. In these same cultures, DJ-1 knock-down did not appreciably affect mitochondrial fission, fusion, or respiration, but did enhance rotenone-induced reductions in the mitochondrial membrane potential. In neuron-astrocyte co-cultures, astrocytic DJ-1 knock-down reduced astrocyte process mitochondrial motility in untreated cells, but this effect was not maintained in the presence of rotenone. In the same co-cultures, astrocytic DJ-1 knock-down significantly reduced mitochondrial fusion in the astrocyte cell bodies, but not the processes, under the same conditions of rotenone treatment in which DJ-1 deficiency is known to impair astrocyte-mediated neuroprotection. Our studies therefore demonstrated the following new findings: (i) DJ-1 deficiency can impair astrocyte mitochondrial physiology at multiple levels, (ii) astrocyte mitochondrial dynamics vary with sub-cellular region, and (iii) the physical presence of neurons can affect astrocyte mitochondrial behavior.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

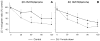


Similar articles
-
DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone.Neurobiol Dis. 2009 Jan;33(1):28-36. doi: 10.1016/j.nbd.2008.09.013. Epub 2008 Sep 30. Neurobiol Dis. 2009. PMID: 18930142 Free PMC article.
-
DJ-1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor-induced neurotoxicity.J Neurochem. 2011 May;117(3):375-87. doi: 10.1111/j.1471-4159.2011.07175.x. Epub 2011 Jan 28. J Neurochem. 2011. PMID: 21219333 Free PMC article.
-
DJ-1 expression modulates astrocyte-mediated protection against neuronal oxidative stress.J Mol Neurosci. 2013 Mar;49(3):507-11. doi: 10.1007/s12031-012-9904-4. Epub 2012 Oct 15. J Mol Neurosci. 2013. PMID: 23065353 Free PMC article.
-
Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease.Exp Neurol. 2009 Aug;218(2):235-46. doi: 10.1016/j.expneurol.2009.03.006. Epub 2009 Mar 18. Exp Neurol. 2009. PMID: 19303005 Review.
-
Mitochondrial dynamics: the intersection of form and function.Adv Exp Med Biol. 2012;748:13-40. doi: 10.1007/978-1-4614-3573-0_2. Adv Exp Med Biol. 2012. PMID: 22729853 Free PMC article. Review.
Cited by
-
SOD1 and DJ-1 converge at Nrf2 pathway: a clue for antioxidant therapeutic potential in neurodegeneration.Oxid Med Cell Longev. 2013;2013:836760. doi: 10.1155/2013/836760. Epub 2013 Jul 28. Oxid Med Cell Longev. 2013. PMID: 23983902 Free PMC article. Review.
-
Products of the Parkinson's disease-related glyoxalase DJ-1, D-lactate and glycolate, support mitochondrial membrane potential and neuronal survival.Biol Open. 2014 Jul 25;3(8):777-84. doi: 10.1242/bio.20149399. Biol Open. 2014. PMID: 25063200 Free PMC article.
-
Neuron-Astrocyte Interactions in Parkinson's Disease.Cells. 2020 Dec 7;9(12):2623. doi: 10.3390/cells9122623. Cells. 2020. PMID: 33297340 Free PMC article. Review.
-
DJ1 represses glycolysis and cell proliferation by transcriptionally up-regulating Pink1.Biochem J. 2015 Apr 15;467(2):303-10. doi: 10.1042/BJ20141025. Biochem J. 2015. PMID: 25670069 Free PMC article.
-
Environmental neurotoxicant-induced dopaminergic neurodegeneration: a potential link to impaired neuroinflammatory mechanisms.Pharmacol Ther. 2019 May;197:61-82. doi: 10.1016/j.pharmthera.2019.01.001. Epub 2019 Jan 22. Pharmacol Ther. 2019. PMID: 30677475 Free PMC article. Review.
References
-
- Ascherio A, Chen H, Weisskopf MG, O’Reilly E, McCullough ML, Calle EE, Schwarzschild MA, Thun MJ. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. - PubMed
-
- Ballard PA, Tetrud JW, Langston JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology. 1985;35:949–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

