Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin
- PMID: 21907282
- PMCID: PMC3225598
- DOI: 10.1016/j.nbd.2011.08.024
Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin
Abstract
Tuberous Sclerosis Complex (TSC) is a multiorgan genetic disease that prominently features brain malformations (tubers) with many patients suffering from epilepsy and autism. These malformations typically exhibit neuronal as well as glial cell abnormalities and likely underlie much of the neurological morbidity seen in TSC. Tuber pathogenesis remains poorly understood though upregulation of the mTORC1 signaling pathway in TSC has been consistently demonstrated. Here we address abnormal brain development in TSC by inactivating the mouse Tsc1 gene in embryonic neural progenitor cells. This strategy permits evaluation of the role of the Tsc1 gene in both neuronal as well as glial cell lineages. Tsc1(Emx1-Cre) conditional knockout (CKO) animals die by 25 days of life. Their brains have increased size and contain prominent large cells within the cerebral cortex that have greatly increased mTORC1 signaling and decreased mTORC2 signaling. Severe defects of cortical lamination, enlarged dysmorphic astrocytes and decreased myelination were also found. Tsc1(Emx1-Cre) CKO mice were then treated with rapamycin to see if the premature death and brain abnormalities can be rescued. Postnatal rapamycin treatment completely prevented premature death and largely reversed the glia pathology but not abnormal neuronal lamination. These findings support a model that loss of function of the TSC genes in embryonic neural progenitor cells causes cortical malformations in patients with TSC. The dramatic effect of rapamycin suggests that even with extensive multi-lineage abnormalities, a postnatal therapeutic window may exist for patients with TSC.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




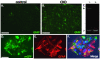
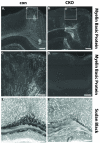
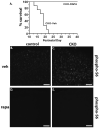


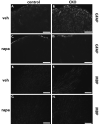
References
-
- Boer K, et al. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 2008;78:7–21. - PubMed
-
- Catania MG, et al. Hamartin expression and interaction with tuberin in tumor cell lines and primary cultures. Journal of Neuroscience Research. 2001;63:276–83. - PubMed
-
- Crino PB. Molecular pathogenesis of tuber formation in tuberous sclerosis complex. J Child Neurol. 2004;19:716–25. - PubMed
-
- Crino PB, et al. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

