Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain
- PMID: 21907491
- PMCID: PMC3199350
- DOI: 10.1016/j.pain.2011.07.020
Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain
Abstract
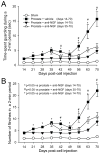
Early, preemptive blockade of nerve growth factor (NGF)/tropomyosin receptor kinase A (TrkA) attenuates tumor-induced nerve sprouting and bone cancer pain. A critical unanswered question is whether late blockade of NGF/TrkA can attenuate cancer pain once NGF-induced nerve sprouting and neuroma formation has occurred. By means of a mouse model of prostate cancer-induced bone pain, anti-NGF was either administered preemptively at day 14 after tumor injection when nerve sprouting had yet to occur, or late at day 35, when extensive nerve sprouting had occurred. Animals were humanely killed at day 70 when, in vehicle-treated animals, significant nerve sprouting and neuroma formation was present in the tumor-bearing bone. Although preemptive and sustained administration (days 14-70) of anti-NGF more rapidly attenuated bone cancer nociceptive behaviors than late and sustained administration (days 35-70), by day 70 after tumor injection, both preemptive and late administration of anti-NGF significantly reduced nociceptive behaviors, sensory and sympathetic nerve sprouting, and neuroma formation. In this model, as in most cancers, the individual cancer cell colonies have a limited half-life because they are constantly proliferating, metastasizing, and undergoing necrosis as the parent cancer cell colony outgrows its blood supply. Similarly, the sensory and sympathetic nerve fibers that innervate the tumor undergo sprouting at the viable/leading edge of the parent tumor, degenerate as the parent cancer cell colony becomes necrotic, and resprout in the viable, newly formed daughter cell colonies. These results suggest that preemptive or late-stage blockade of NGF/TrkA can attenuate nerve sprouting and cancer pain.
Copyright © 2011 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None of the authors of this study claim a conflict of interest.
Figures






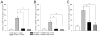

Comment in
-
Clinical perspectives on preclinical models of bone pain: questions and promises.Pain. 2011 Nov;152(11):2455-2456. doi: 10.1016/j.pain.2011.08.008. Epub 2011 Aug 24. Pain. 2011. PMID: 21868166 No abstract available.
References
-
- Adriaenssens E, Vanhecke E, Saule P, Mougel A, Page A, Romon R, Nurcombe V, Le Bourhis X, Hondermarck H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008;68(2):346–351. - PubMed
-
- Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, Rowley D. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49(3):213–223. - PubMed
-
- Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol. 2008;64(6):644–653. - PubMed
-
- Bloom RA, Libson E, Husband JE, Stoker DJ. The periosteal sunburst reaction to bone metastases. A literature review and report of 20 additional cases. Skeletal Radiol. 1987;16(8):629–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

