Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels
- PMID: 21907716
- PMCID: PMC3195874
- DOI: 10.1016/j.ygcen.2011.08.019
Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels
Abstract
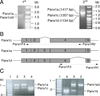
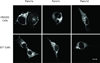
Our previous studies have showed that Pannexin 1 (Panx1), a member of a recently discovered family of gap junction proteins, is expressed in the pituitary gland. Here we investigated the presence and expression pattern of Panx1 isoforms in pituitary cells, their roles in ATP release, and their association with purinergic P2X receptor subtypes that are native to pituitary cells. In addition to the full-size Panx1, termed Panx1a, pituitary cells also express two novel shorter isoforms, termed Panx1c and Panx1d, which formation reflects the existence of alternative splicing sites in exons 2 and 4, respectively. Panx1c is lacking the Phe108-Gln180 sequence and P2X1d is missing the Val307-Cys426 C-terminal end sequence. Confocal microscopy and biotin labeling revealed that Panx1a is expressed in the plasma membrane, whereas Panx1c and Panx1d show the cytoplasmic localization when expressed as homomeric proteins. The three Panx1 isoforms and Panx2 form homomeric and heteromeric complexes in any combination. These splice forms can also physically associate with ATP-gated P2X2, P2X3, P2X4, and P2X7 receptor channels. The Panx1a-mediated ATP release in AtT-20 immortalized pituitary cells is attenuated when co-expressed with Panx1c or Panx1d. These results suggest that Panx1c and Panx1d may serve as dominant-negative effectors to modulate the functions of Panx1a through formation of heteromeric channels. The complex patterns of Panx1 expression and association could also define the P2X-dependent roles of these channels in cell types co-expressing both proteins.
Published by Elsevier Inc.
Figures






References
-
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. - PubMed
-
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. - PubMed
-
- Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 2006;21:103–114. - PubMed
-
- Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. - PubMed
-
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

