Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial
- PMID: 21908036
- PMCID: PMC4151875
- DOI: 10.1016/S0140-6736(11)61383-4
Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial
Abstract
Background: Dalcetrapib modulates cholesteryl ester transfer protein (CETP) activity to raise high-density lipoprotein cholesterol (HDL-C). After the failure of torcetrapib it was unknown if HDL produced by interaction with CETP had pro-atherogenic or pro-inflammatory properties. dal-PLAQUE is the first multicentre study using novel non-invasive multimodality imaging to assess structural and inflammatory indices of atherosclerosis as primary endpoints.
Methods: In this phase 2b, double-blind, multicentre trial, patients (aged 18-75 years) with, or with high risk of, coronary heart disease were randomly assigned (1:1) to dalcetrapib 600 mg/day or placebo for 24 months. Randomisation was done with a computer-generated randomisation code and was stratified by centre. Patients and investigators were masked to treatment. Coprimary endpoints were MRI-assessed indices (total vessel area, wall area, wall thickness, and normalised wall index [average carotid]) after 24 months and (18)F-fluorodeoxyglucose ((18)F-FDG) PET/CT assessment of arterial inflammation within an index vessel (right carotid, left carotid, or ascending thoracic aorta) after 6 months, with no-harm boundaries established before unblinding of the trial. Analysis was by intention to treat. This trial is registered at ClinicalTrials.gov, NCT00655473.
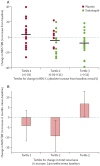
Findings: 189 patients were screened and 130 randomly assigned to placebo (66 patients) or dalcetrapib (64 patients). For the coprimary MRI and PET/CT endpoints, CIs were below the no-harm boundary or the adverse change was numerically lower in the dalcetrapib group than in the placebo group. MRI-derived change in total vessel area was reduced in patients given dalcetrapib compared with those given placebo after 24 months; absolute change from baseline relative to placebo was -4·01 mm(2) (90% CI -7·23 to -0·80; nominal p=0·04). The PET/CT measure of index vessel most-diseased-segment target-to-background ratio (TBR) was not different between groups, but carotid artery analysis showed a 7% reduction in most-diseased-segment TBR in the dalcetrapib group compared with the placebo group (-7·3 [90% CI -13·5 to -0·8]; nominal p=0·07). Dalcetrapib did not increase office blood pressure and the frequency of adverse events was similar between groups.
Interpretation: Dalcetrapib showed no evidence of a pathological effect related to the arterial wall over 24 months. Moreover, this trial suggests possible beneficial vascular effects of dalcetrapib, including the reduction in total vessel enlargement over 24 months, but long-term safety and clinical outcomes efficacy of dalcetrapib need to be analysed.
Funding: F Hoffmann-La Roche Ltd.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
VM and VF declare that they have no conflicts of interest.
Figures


Comment in
-
Dalcetrapib: turning the tide for CETP inhibition?Lancet. 2011 Oct 29;378(9802):1529-30. doi: 10.1016/S0140-6736(11)61421-9. Epub 2011 Sep 9. Lancet. 2011. PMID: 21908039 No abstract available.
-
Coronary artery disease: Dalcetrapib safely raises HDL-cholesterol level in the phase IIb dal-PLAQUE trial.Nat Rev Cardiol. 2011 Sep 27;8(11):610. doi: 10.1038/nrcardio.2011.150. Nat Rev Cardiol. 2011. PMID: 21946777 No abstract available.
-
Dalcetrapib--restoring belief in modulating CETP as a beneficial mechanism in cardiovascular disease.Expert Opin Investig Drugs. 2012 Apr;21(4):569-73. doi: 10.1517/13543784.2012.659817. Epub 2012 Feb 13. Expert Opin Investig Drugs. 2012. PMID: 22329439
References
-
- Scandinavian Simvastatin Survival study group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival study (4S) Lancet. 1994;344:1383–89. - PubMed
-
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–04. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

