Targeting the internal epitope of prostate-specific membrane antigen with 89Zr-7E11 immuno-PET
- PMID: 21908391
- PMCID: PMC3537833
- DOI: 10.2967/jnumed.111.092098
Targeting the internal epitope of prostate-specific membrane antigen with 89Zr-7E11 immuno-PET
Abstract
The potential of the positron-emitting (89)Zr has been recently investigated for the design of radioimmunoconjugates for immuno-PET. In this study, we report the preparation and in vivo evaluation of (89)Zr-desferrioxamine B (DFO)-7E11, a novel (89)Zr-labeled monoclonal antibody (mAb) construct for targeted imaging of prostate-specific membrane antigen (PSMA), a prototypical cell surface marker highly overexpressed in prostate cancer. The ability of (89)Zr-DFO-7E11 to delineate tumor response to therapy was also investigated, because it binds to the intracellular epitope of PSMA, which becomes available only on membrane disruption in dead or dying cells.
Methods: 7E11 as a marker of dying cells was studied by flow cytometry and microscopy of cells after antiandrogen-, radio-, and chemotherapy in LNCaP and PC3 PSMA-positive cells. The in vivo behavior of (89)Zr-DFO-7E11 was characterized in mice bearing subcutaneous LNCaP (PSMA-positive) tumors by biodistribution studies and immuno-PET. The potential of assessing tumor response was evaluated in vivo after radiotherapy.
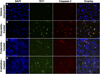
Results: In vitro studies correlated 7E11 binding with markers of apoptosis (7-amino-actinomycin-D and caspase-3). In vivo biodistribution experiments revealed high, target-specific uptake of (89)Zr-DFO-7E11 in LNCaP tumors after 24 h (20.35 ± 7.50 percentage injected dose per gram [%ID/g]), 48 h (22.82 ± 3.58 %ID/g), 96 h (36.94 ± 6.27 %ID/g), and 120 h (25.23 ± 4.82 %ID/g). Excellent image contrast was observed with immuno-PET. 7E11 uptake was statistically increased in irradiated versus control tumor as measured by immuno-PET and biodistribution studies. Binding specificity was assessed by effective blocking studies at 48 h.
Conclusion: These findings suggest that (89)Zr-DFO-7E11 displays high tumor-to-background tissue contrast in immuno-PET and can be used as a tool to monitor and quantify, with high specificity, tumor response in PSMA-positive prostate cancer.
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures


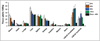

References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Attard G, de Bono JS. Prostate cancer: PSA as an intermediate end point in clinical trials. Nat Rev Urol. 2009;6:473–475. - PubMed
-
- Liu IJ, Zafar MB, Lai YH, Segall GM, Terris MK. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organconfined prostate cancer. Urology. 2001;57:108–111. - PubMed
-
- Oyama N, Akino H, Suzuki Y, et al. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
