Breast cancer subtype-specific interactions with the microenvironment dictate mechanisms of invasion
- PMID: 21908556
- PMCID: PMC3206184
- DOI: 10.1158/0008-5472.CAN-11-1818
Breast cancer subtype-specific interactions with the microenvironment dictate mechanisms of invasion
Abstract
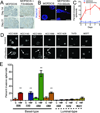
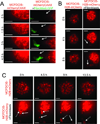
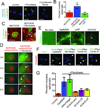
Most ductal breast carcinoma cells are weakly invasive in vitro and in vivo, suggesting that components of their microenvironment may facilitate a transition from in situ to invasive stages during progression. Here, we report that coculture of mammary fibroblasts specifically triggers invasive behavior in basal-type breast cancer cells through a ligand independent mechanism. When cultured alone in organotypic culture, both basal- and luminal-type breast cancer cells formed noninvasive spheroids with characteristics of ductal carcinoma in situ (DCIS). In contrast, when cocultured with mammary fibroblasts, basal-type spheroids exhibited invasive character whereas the luminal-type spheroids retained a benign and noninvasive duct-like architecture. Real-time imaging and functional studies revealed that the specificity of invasion was linked to a unique capacity of basal-type breast cancer cells to move within spheroids. Mammary fibroblasts induced invasion by triggering basal-type breast cancer cells to convert from a noninvasive program of mammary epithelial morphogenesis to an invasive program of sprouting endothelial angiogenesis. Contrary to the existing invasion models, soluble ligands produced by the fibroblasts were not sufficient to trigger invasion. Instead, basal-type invasion relied upon a Cdc42-dependent reorganization of collagen fibers in the extracellular matrix by fibroblasts. Inhibiting basal-type cell movement with clinically relevant drugs blocked invasion both in organotypic culture and in animals, suggesting a new treatment strategy for early-stage patients. Together our findings establish that fibroblast recruitment by basal-type breast cancer cells into early-stage tumors is sufficient to trigger their conversion from a benign, noninvasive DCIS-like stage to a malignant invasive stage. Furthermore, our findings suggest that different subtypes of breast cancer may require distinct types of contributions from the microenvironment to undergo malignant progression.
©2011 AACR.
Figures


References
-
- Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–1441. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

