Molecular coupling in the human ether-a-go-go-related gene-1 (hERG1) K+ channel inactivation pathway
- PMID: 21908602
- PMCID: PMC3234734
- DOI: 10.1074/jbc.M111.292060
Molecular coupling in the human ether-a-go-go-related gene-1 (hERG1) K+ channel inactivation pathway
Abstract
Emerging evidence suggests that K(+) channel inactivation involves coupling between residues in adjacent regions of the channel. Human ether-a-go-go-related gene-1 (hERG1) K(+) channels undergo a fast inactivation gating process that is crucial for maintaining electrical stability in the heart. The molecular mechanisms that drive inactivation in hERG1 channels are unknown. Using alanine scanning mutagenesis, we show that a pore helix residue (Thr-618) that points toward the S5 segment is critical for normal inactivation gating. Amino acid substitutions at position 618 modulate the free energy of inactivation gating, causing enhanced or reduced inactivation. Mutation of an S5 residue that is predicted to be adjacent to Thr-618 (W568L) abolishes inactivation and alters ion selectivity. The introduction of the Thr-618-equivalent residue in Kv1.5 enhances inactivation. Molecular dynamic simulations of the Kv1.2 tetramer reveal van der Waals coupling between hERG1 618- and 568-equivalent residues and a significant increase in interaction energies when threonine is introduced at the 618-equivalent position. We propose that coupling between the S5 segment and pore helix may participate in the inactivation process in hERG1 channels.
Figures




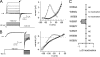
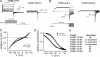
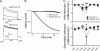
References
-
- Huffaker S. J., Chen J., Nicodemus K. K., Sambataro F., Yang F., Mattay V., Lipska B. K., Hyde T. M., Song J., Rujescu D., Giegling I., Mayilyan K., Proust M. J., Soghoyan A., Caforio G., Callicott J. H., Bertolino A., Meyer-Lindenberg A., Chang J., Ji Y., Egan M. F., Goldberg T. E., Kleinman J. E., Lu B., Weinberger D. R. (2009) Nat. Med. 15, 509–518 - PMC - PubMed
-
- Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. (1995) Cell 81, 299–307 - PubMed
-
- Wang H., Zhang Y., Cao L., Han H., Wang J., Yang B., Nattel S., Wang Z. (2002) Cancer Res. 62, 4843–4848 - PubMed
-
- Trudeau M. C., Warmke J. W., Ganetzky B., Robertson G. A. (1995) Science 269, 92–95 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

