Collagen VI, conformation of A-domain arrays and microfibril architecture
- PMID: 21908605
- PMCID: PMC3220584
- DOI: 10.1074/jbc.M111.265595
Collagen VI, conformation of A-domain arrays and microfibril architecture
Abstract
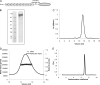
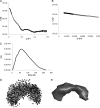
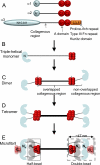
Collagen VI is a ubiquitous extracellular matrix protein that assembles into beaded microfibrils that form networks linking cells to the matrix. Collagen VI microfibrils are typically formed from a heterotrimer of the α1, α2, and α3 chains. The α3 chain is distinct as it contains an extended N terminus with up to 10 consecutive von Willebrand factor type A-domains (VWA). Here, we use solution small angle x-ray scattering (SAXS) and single particle analysis EM to determine the nanostructure of nine of these contiguous A-domains. Both techniques reveal a tight C-shape conformation for the A-domains. Furthermore, using biophysical approaches, we demonstrate that the N-terminal region undergoes a conformational change and a proportion forms dimers in the presence of Zn(2+). This is the first indication that divalent cations interact with collagen VI A-domains. A three-dimensional reconstruction of tissue-purified collagen VI microfibrils was generated using EM and single particle image analysis. The reconstruction showed the intricate architecture of the collagen VI globular regions, in particular the highly structurally conserved C-terminal region and variations in the appearance of the N-terminal region. The N-terminal domains project out from the globular beaded region like angled radial spokes. These could potentially provide interactive surfaces for other cell matrix molecules.
Figures





References
-
- Gara S. K., Grumati P., Urciuolo A., Bonaldo P., Kobbe B., Koch M., Paulsson M., Wagener R. (2008) J. Biol. Chem. 283, 10658–10670 - PubMed
-
- Fitzgerald J., Rich C., Zhou F. H., Hansen U. (2008) J. Biol. Chem. 283, 20170–20180 - PubMed
-
- Baker N. L., Mörgelin M., Peat R., Goemans N., North K. N., Bateman J. F., Lamandé S. R. (2005) Hum. Mol. Genet. 14, 279–293 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/D008662/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBSSC200513125/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT081406MA/WT_/Wellcome Trust/United Kingdom
- D008662/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

