The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis
- PMID: 21908668
- PMCID: PMC3194919
- DOI: 10.1128/JB.05872-11
The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis
Abstract
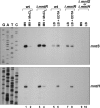
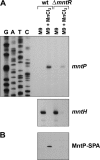
Manganese is a critical micronutrient for cells, serving as an enzyme cofactor and protecting against oxidative stress. Yet, manganese is toxic in excess and little is known about its distribution in cells. Bacteria control intracellular manganese levels by the transcription regulator MntR. When this work began, the only Escherichia coli K-12 gene known to respond to manganese via MntR repression was mntH, which encodes a manganese importer. We show that mntS (formerly the small RNA gene rybA) is repressed by manganese through MntR and encodes an unannotated 42-amino-acid protein. Overproduction of MntS causes manganese sensitivity, while a lack of MntS perturbs proper manganese-dependent repression of mntH. We also provide evidence that mntP (formerly yebN), which encodes a putative efflux pump, is positively regulated by MntR. Deletion of mntP leads to profound manganese sensitivity and to elevated intracellular manganese levels. This work thus defines two new proteins involved in manganese homeostasis and suggests mechanisms for their action.
Figures






References
-
- Cherepanov P. P., Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14 - PubMed
-
- Cromie M. J., Shi Y., Latifi T., Groisman E. A. 2006. An RNA sensor for intracellular Mg2+. Cell 125:71–84 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

