Restoring soluble guanylyl cyclase expression and function blocks the aggressive course of glioma
- PMID: 21908708
- PMCID: PMC3228529
- DOI: 10.1124/mol.111.073585
Restoring soluble guanylyl cyclase expression and function blocks the aggressive course of glioma
Abstract
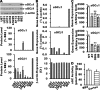
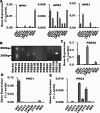
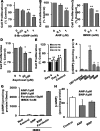
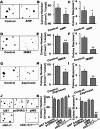
The NO and cGMP signaling pathways are of broad physiological and pathological significance. We compared the NO/soluble guanylyl cyclase (sGC)/cGMP pathway in human glioma tissues and cell lines with that of healthy control samples and demonstrated that sGC expression is significantly lower in glioma preparations. Our analysis of GEO databases (National Cancer Institute) further revealed a statistically significant reduction of sGC transcript levels in human glioma specimens. On the other hand, the expression levels of particulate (membrane) guanylyl cyclases (pGC) and cGMP-specific phosphodiesterase (PDE) were intact in the glioma cells that we have tested. Pharmacologically manipulating endogenous cGMP generation in glioma cells through either stimulating pGC by ANP/BNP, or blocking PDE by 3-isobutyl-1-methylxanthine/zaprinast caused significant inhibition of proliferation and colony formation of glioma cells. Genetically restoring sGC expression also correlated inversely with glioma cells growth. Orthotopic implantation of glioma cells transfected with an active mutant form of sGC (sGCα1β1(Cys105)) in athymic mice increased the survival time by 4-fold over the control. Histological analysis of xenografts overexpressing α1β1(Cys105) sGC revealed changes in cellular architecture that resemble the morphology of normal cells. In addition, a decrease in angiogenesis contributed to glioma inhibition by sGC/cGMP therapy. Our study proposes the new concept that suppressed expression of sGC, a key enzyme in the NO/cGMP pathway, may be associated with an aggressive course of glioma. The sGC/cGMP signaling-targeted therapy may be a favorable alternative to chemotherapy and radiotherapy for glioma and perhaps other tumors.
Figures



References
-
- Basson MD, Hong F. (1996) Regulation of human Caco-2 intestinal epithelial brush border enzyme activity by cyclic nucleotides. Cancer Lett 99:155–160 - PubMed
-
- Bian K, Harari Y, Zhong M, Lai M, Castro G, Weisbrodt N, Murad F. (2001) Down-regulation of inducible nitric-oxide synthase (NOS-2) during parasite-induced gut inflammation: a path to identify a selective NOS-2 inhibitor. Mol Pharmacol 59:939–947 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

