The first identification of lysine malonylation substrates and its regulatory enzyme
- PMID: 21908771
- PMCID: PMC3237090
- DOI: 10.1074/mcp.M111.012658
The first identification of lysine malonylation substrates and its regulatory enzyme
Abstract
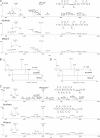
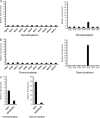
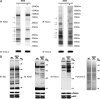
Protein post-translational modifications (PTMs) at the lysine residue, such as lysine methylation, acetylation, and ubiquitination, are diverse, abundant, and dynamic. They play a key role in the regulation of diverse cellular physiology. Here we report discovery of a new type of lysine PTM, lysine malonylation (Kmal). Kmal was initially detected by mass spectrometry and protein sequence-database searching. The modification was comprehensively validated by Western blot, tandem MS, and high-performance liquid chromatography of synthetic peptides, isotopic labeling, and identification of multiple Kmal substrate proteins. Kmal is a dynamic and evolutionarily conserved PTM observed in mammalian cells and bacterial cells. In addition, we demonstrate that Sirt5, a member of the class III lysine deacetylases, can catalyze lysine demalonylation and lysine desuccinylation reactions both in vitro and in vivo. This result suggests the possibility of nondeacetylation activity of other class III lysine deacetylases, especially those without obvious acetylation protein substrates. Our results therefore reveal a new type of PTM pathway and identify the first enzyme that can regulate lysine malonylation and lysine succinylation status.
Figures




References
-
- Witze E. S., Old W. M., Resing K. A., Ahn N. G. (2007) Mapping protein post-translational modifications with mass spectrometry. Nat. Methods 4, 798–806 - PubMed
-
- Walsh C. T., Garneau-Tsodikova S., Gatto G. J., Jr. (2005) Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem. Int. Ed Engl. 44, 7342–7372 - PubMed
-
- Johnson S. A., Hunter T. (2005) Kinomics: methods for deciphering the kinome. Nat. Methods 2, 17–25 - PubMed
-
- Denu J. M., Dixon J. E. (1998) Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Curr. Opin. Chem. Biol. 2, 633–641 - PubMed
-
- Guarente L. (2007) Sirtuins in aging and disease. Cold Spring Harb. Symp Quant Biol. 72, 483–488 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

