General Anesthesia Causes Long-term Impairment of Mitochondrial Morphogenesis and Synaptic Transmission in Developing Rat Brain
- PMID: 21909020
- PMCID: PMC3203321
- DOI: 10.1097/ALN.0b013e3182303a63
General Anesthesia Causes Long-term Impairment of Mitochondrial Morphogenesis and Synaptic Transmission in Developing Rat Brain
Abstract
Background: Clinically used general anesthetics, alone or in combination, are damaging to the developing mammalian brain. In addition to causing widespread apoptotic neurodegeneration in vulnerable brain regions, exposure to general anesthesia at the peak of synaptogenesis causes learning and memory deficiencies later in life. In vivo rodent studies have suggested that activation of the intrinsic (mitochondria-dependent) apoptotic pathway is the earliest warning sign of neuronal damage, suggesting that a disturbance in mitochondrial integrity and function could be the earliest triggering events.
Methods: Because proper and timely mitochondrial morphogenesis is critical for brain development, the authors examined the long-term effects of a commonly used anesthesia combination (isoflurane, nitrous oxide, and midazolam) on the regional distribution, ultrastructural properties, and electron transport chain function of mitochondria, as well as synaptic neurotransmission, in the subiculum of rat pups.
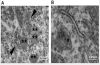
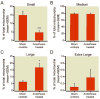
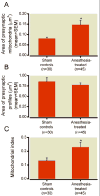
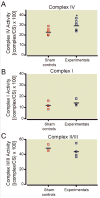
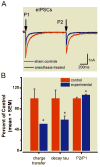
Results: This anesthesia, administered at the peak of synaptogenesis, causes protracted injury to mitochondria, including significant enlargement of mitochondria (more than 30%, P < 0.05), impairment of their structural integrity, an approximately 28% increase in their complex IV activity (P < 0.05), and a twofold decrease in their regional distribution in presynaptic neuronal profiles (P < 0.05), where their presence is important for the normal development and functioning of synapses. Consequently, the authors showed that impaired mitochondrial morphogenesis is accompanied by heightened autophagic activity, decrease in mitochondrial density (approximately 27%, P < 0.05), and long-lasting disturbances in inhibitory synaptic neurotransmission. The interrelation of these phenomena remains to be established.
Conclusion: Developing mitochondria are exquisitely vulnerable to general anesthesia and may be important early target of anesthesia-induced developmental neurodegeneration.
Figures



References
-
- Yon J-H, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;35:815–27. - PubMed
-
- Yon J-H, Carter LB, Reiter RJ, Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis. 2006;21:522–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

