Matrix-embedded cells control osteoclast formation
- PMID: 21909103
- PMCID: PMC3192296
- DOI: 10.1038/nm.2448
Matrix-embedded cells control osteoclast formation
Abstract
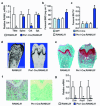
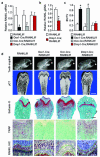
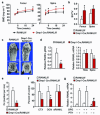
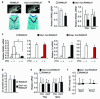
Osteoclasts resorb the mineralized matrices formed by chondrocytes or osteoblasts. The cytokine receptor activator of nuclear factor-κB ligand (RANKL) is essential for osteoclast formation and thought to be supplied by osteoblasts or their precursors, thereby linking bone formation to resorption. However, RANKL is expressed by a variety of cell types, and it is unclear which of them are essential sources for osteoclast formation. Here we have used a mouse strain in which RANKL can be conditionally deleted and a series of Cre-deleter strains to demonstrate that hypertrophic chondrocytes and osteocytes, both of which are embedded in matrix, are essential sources of the RANKL that controls mineralized cartilage resorption and bone remodeling, respectively. Moreover, osteocyte RANKL is responsible for the bone loss associated with unloading. Contrary to the current paradigm, RANKL produced by osteoblasts or their progenitors does not contribute to adult bone remodeling. These results suggest that the rate-limiting step of matrix resorption is controlled by cells embedded within the matrix itself.
Figures
Comment in
-
Osteocytes, RANKL and bone loss.Nat Rev Endocrinol. 2011 Oct 4;7(12):693. doi: 10.1038/nrendo.2011.176. Nat Rev Endocrinol. 2011. PMID: 21970841 No abstract available.
-
Bone: Osteocyte RANKL in bone homeostasis: a paradigm shift?Nat Rev Rheumatol. 2011 Oct 11;7(11):619. doi: 10.1038/nrrheum.2011.151. Nat Rev Rheumatol. 2011. PMID: 21989285 No abstract available.
References
-
- Kronenberg HM. Developmental regulation of the growth plate [Review] Nature. 2003;423:332–336. - PubMed
-
- Parfitt AM. Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone. 2002;30:5–7. - PubMed
-
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4:638–649. - PubMed
-
- Whyte MP, et al. Osteoprotegerin deficiency and juvenile Paget’s disease. N. Engl. J. Med. 2002;347:175–184. - PubMed
-
- Manolagas SC. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis [Review] Endocr. Rev. 2000;21:115–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

