Glutamate release by primary brain tumors induces epileptic activity
- PMID: 21909104
- PMCID: PMC3192231
- DOI: 10.1038/nm.2453
Glutamate release by primary brain tumors induces epileptic activity
Abstract
Epileptic seizures are a common and poorly understood comorbidity for individuals with primary brain tumors. To investigate peritumoral seizure etiology, we implanted human-derived glioma cells into severe combined immunodeficient mice. Within 14-18 d, glioma-bearing mice developed spontaneous and recurring abnormal electroencephalogram events consistent with progressive epileptic activity. Acute brain slices from these mice showed marked glutamate release from the tumor mediated by the system x(c)(-) cystine-glutamate transporter (encoded by Slc7a11). Biophysical and optical recordings showed glutamatergic epileptiform hyperexcitability that spread into adjacent brain tissue. We inhibited glutamate release from the tumor and the ensuing hyperexcitability by sulfasalazine (SAS), a US Food and Drug Administration-approved drug that blocks system x(c)(-). We found that acute administration of SAS at concentrations equivalent to those used to treat Crohn's disease in humans reduced epileptic event frequency in tumor-bearing mice compared with untreated controls. SAS should be considered as an adjuvant treatment to ameliorate peritumoral seizures associated with glioma in humans.
Conflict of interest statement
The authors declare no competing conflicts of interest.
Figures

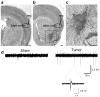
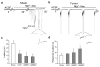


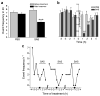
Comment in
-
Glioma-related seizures: glutamate is the key.Nat Med. 2011 Oct 11;17(10):1190-1. doi: 10.1038/nm.2510. Nat Med. 2011. PMID: 21988994 No abstract available.
References
-
- Moots PL, et al. The course of seizure disorders in patients with malignant gliomas. Arch Neurol. 1995;52 :717–724. - PubMed
-
- van Breemen MS, et al. Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol. 2009;256:1519–1526. - PubMed
-
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. - PubMed
-
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. - PubMed
-
- Patt S, et al. Source localization and possible causes of interictal epileptic activity in tumor-associated epilepsy. Neurobiol Dis. 2000;7:260–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 HD038985/HD/NICHD NIH HHS/United States
- 2R01-NS052634/NS/NINDS NIH HHS/United States
- T32 NS048039/NS/NINDS NIH HHS/United States
- 5R01-NS036692/NS/NINDS NIH HHS/United States
- P50 CA097247/CA/NCI NIH HHS/United States
- U54 CA 100949/CA/NCI NIH HHS/United States
- R01 NS052634/NS/NINDS NIH HHS/United States
- P30 DK079337/DK/NIDDK NIH HHS/United States
- S10 RR19231/RR/NCRR NIH HHS/United States
- P30 NS057098/NS/NINDS NIH HHS/United States
- P50-CA097247/CA/NCI NIH HHS/United States
- S10 RR019231/RR/NCRR NIH HHS/United States
- NS57098/NS/NINDS NIH HHS/United States
- P50 AT00477/AT/NCCIH NIH HHS/United States
- P50 AT000477/AT/NCCIH NIH HHS/United States
- 5T32NS048039-03/NS/NINDS NIH HHS/United States
- P30 AR50948/AR/NIAMS NIH HHS/United States
- R01 NS036692/NS/NINDS NIH HHS/United States
- U54 CA100949/CA/NCI NIH HHS/United States
- P30 AR050948/AR/NIAMS NIH HHS/United States

