CDR3δ -grafted γ9δ2T cells mediate effective antitumor reactivity
- PMID: 21909128
- PMCID: PMC4002801
- DOI: 10.1038/cmi.2011.28
CDR3δ -grafted γ9δ2T cells mediate effective antitumor reactivity
Abstract
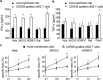
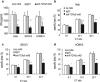
Adoptive cell-transfer therapy (ACT) has been reported to suppress growing tumors and to overcome tumor escape in animal models. As a candidate ACT effector, γ9δ2T cells can be activated and expanded in vitro and in vivo and display strong antitumor activity against colorectal, lung, prostate, ovarian and renal cell carcinomas. However, it is difficult to obtain a large enough number of γδT cells to meet the need for immunotherapy that can overcome the cancer patients' immune suppressive tumor microenvironment. In previous studies, our lab confirmed that γ9δ2T cells recognized tumor cells via the CDR3δ region of the γδ-T-cell receptor (TCR). We constructed full-length human peripheral blood mononuclear cell (PBMC)-derived γ9 and δ2 chains in which the CDR3 region was replaced by an ovarian epithelial carcinoma (OEC)-derived CDR3. We transferred the CDR3δ-grafted γ9δ2TCR into peripheral blood lymphocytes (PBLs) to develop genetically modified γ9δ2T cells. In vitro studies have shown that these CDR3δ-grafted γ9δ2T cells can produce cytokines after stimulation with tumor cell extracts and exhibit cytotoxicity towards tumor cells, including human OEC and cervical adenocarcinoma. CDR3δ-grafted γ9δ2T cells adoptively transferred into nude mice bearing a human OEC cell line demonstrated significant antitumor effects. These results indicate that CDR3δ-grafted γ9δ2T cells might be candidates for clinical tumor immunotherapy.
Figures



Similar articles
-
Profiling the pattern of the human T-cell receptor γδ complementary determinant region 3 repertoire in patients with lung carcinoma via high-throughput sequencing analysis.Cell Mol Immunol. 2019 Mar;16(3):250-259. doi: 10.1038/cmi.2017.157. Epub 2018 Feb 5. Cell Mol Immunol. 2019. PMID: 30886423 Free PMC article.
-
Targeting solid tumors via T cell receptor complementarity-determining region 3delta in an engineered antibody.Cancer Lett. 2008 Dec 18;272(2):242-52. doi: 10.1016/j.canlet.2008.07.015. Epub 2008 Sep 7. Cancer Lett. 2008. PMID: 18782650
-
γ9 and δ2CDR3 domains regulate functional avidity of T cells harboring γ9δ2TCRs.Blood. 2012 Dec 20;120(26):5153-62. doi: 10.1182/blood-2012-05-432427. Epub 2012 Sep 27. Blood. 2012. PMID: 23018643
-
γδ T cells in cancer immunotherapy.Oncotarget. 2017 Jan 31;8(5):8900-8909. doi: 10.18632/oncotarget.13051. Oncotarget. 2017. PMID: 27823972 Free PMC article. Review.
-
Engineering T cells for adoptive therapy: outsmarting the tumor.Curr Opin Immunol. 2018 Apr;51:133-139. doi: 10.1016/j.coi.2018.03.014. Epub 2018 Mar 24. Curr Opin Immunol. 2018. PMID: 29579622 Review.
Cited by
-
Vγ9Vδ2-T lymphocytes have impaired antiviral function in small-for-gestational-age and preterm neonates.Cell Mol Immunol. 2013 May;10(3):253-60. doi: 10.1038/cmi.2012.78. Epub 2013 Mar 25. Cell Mol Immunol. 2013. PMID: 23524656 Free PMC article.
-
Current Advances in γδ T Cell-Based Tumor Immunotherapy.Front Immunol. 2017 Oct 27;8:1401. doi: 10.3389/fimmu.2017.01401. eCollection 2017. Front Immunol. 2017. PMID: 29163482 Free PMC article. Review.
-
The Generation of Human γδT Cell-Derived Induced Pluripotent Stem Cells from Whole Peripheral Blood Mononuclear Cell Culture.Stem Cells Transl Med. 2018 Jan;7(1):34-44. doi: 10.1002/sctm.17-0021. Epub 2017 Nov 21. Stem Cells Transl Med. 2018. PMID: 29164800 Free PMC article.
-
Trial Watch: Adoptive cell transfer for anticancer immunotherapy.Oncoimmunology. 2013 May 1;2(5):e24238. doi: 10.4161/onci.24238. Oncoimmunology. 2013. PMID: 23762803 Free PMC article.
-
Role of Immune Cells and Receptors in Cancer Treatment: An Immunotherapeutic Approach.Vaccines (Basel). 2022 Sep 7;10(9):1493. doi: 10.3390/vaccines10091493. Vaccines (Basel). 2022. PMID: 36146572 Free PMC article. Review.
References
-
- Peng L, Shu SKrauss JC. Treatment of subcutaneous tumor with adoptively transferred T cells. Cell Immunol. 1997;178:24–32. - PubMed
-
- Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. - PubMed
-
- Plautz GE, Bukowski RM, Novick AC, Klein EA, Kursh ED, Olencki TE, et al. T-cell adoptive immunotherapy of metastatic renal cell carcinoma Urology 199954617–623.discussion 623–614. - PubMed
-
- Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

